Transplantation-Associated Thrombotic Microangiopathy Risk Stratification: Is There a Window of Opportunity to Improve Outcomes?
- PMID: 35490975
- PMCID: PMC9351710
- DOI: 10.1016/j.jtct.2022.04.019
Transplantation-Associated Thrombotic Microangiopathy Risk Stratification: Is There a Window of Opportunity to Improve Outcomes?
Abstract
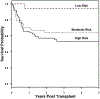
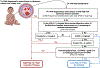
Transplantation-associated thrombotic microangiopathy (TA-TMA) can range from a self-limiting condition to a lethal transplantation complication. It is important to identify TA-TMA patients at risk for severe multiorgan endothelial injury to implement targeted therapies in a timely manner. Current therapeutic approaches with complement blockade have improved survival markedly in high-risk TA-TMA patients, yet one-third of these patients respond inadequately to eculizumab therapy. Poor response may indicate that substantial endothelial injury has already occurred and raises the possibility that earlier intervention may improve outcomes. The goal of this study was to identify additional TA-TMA patients who would benefit from early targeted intervention and update TA-TMA risk stratification methods to reflect these findings. We studied 130 HSCT recipients with a diagnosis of TA-TMA who were screened prospectively and stratified into 3 TA-TMA risk groups (high-risk, n = 64; moderate-risk, n = 48; 18 low-risk, n = 18). We specifically examined TA-TMA biomarkers and clinical outcomes in subjects who were not offered complement blocking therapy (moderate-risk and low-risk TA-TMA subjects) and compared them with those who received TA-TMA-targeted therapy (high-risk TA-TMA subjects). One-year post-HSCT survival for subjects with untreated moderate-risk TA-TMA was similar to those with high-risk TA-TMA receiving eculizumab therapy (71% versus 66%; P = .40), indicating that a subset of moderate-risk patients may benefit from therapy. A detailed analysis of moderate-risk subjects highlighted the importance of relative as well as absolute complement pathway activation in determining organ injury. We demonstrated that activated terminal complement (measured by elevated blood sC5b-9) alone is a valuable indicator of reduced survival. Moderate-risk TA-TMA subjects with elevated sC5b-9 levels had a nearly 3-fold higher risk of mortality that was statistically significant in multivariant analyses (P = .01). A "dose effect" also was observed, and higher sC5b-9 levels were associated with worse outcomes. Furthermore, all moderate-risk patients with sustained sC5b-9 elevation for >2 weeks ultimately developed multiorgan dysfunction syndrome (MODS). This indicates that scheduled sC5b-9 measurements could promptly identify patients at risk for poor outcomes and would facilitate early TA-TMA-directed therapy to prevent organ injury. Untreated low-risk TA-TMA patients had a 1-year post-HSCT survival of 94% and should be observed without targeted interventions. Routine TA-TMA screening and complement-blocking therapies have markedly improved the outcomes for high-risk TA-TMA patients, and our study suggests that additional patients may benefit from TA-TMA treatment. This study provides further support for prospective TA-TMA screening as an integral tool for identifying patients at greatest risk for organ injury and death from TA-TMA. An updated TA-TMA risk algorithm that incorporates relevant laboratory biomarkers, clinical findings, and comorbid conditions was generated using this study's findings, and we propose clinical implementation of this algorithm for the management of TA-TMA.
Keywords: Complement; Eculizumab; HSCT; TA-TMA risk stratification; Transplantation-associated thrombotic microangiopathy.
Copyright © 2022 The American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Moiseev IS, Tsvetkova T, Aljurf M, et al. Clinical and morphological practices in the diagnosis of transplant-associated microangiopathy: a study on behalf of Transplant Complications Working Party of the EBMT. Bone Marrow Transplant. 2019;54:1022–1028. - PubMed
-
- Jodele S, Sabulski A. Transplant-associated thrombotic microangiopathy: elucidating prevention strategies and identifying high-risk patients. Expert Rev Hematol. 2021;14:751–763. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

