Mechanical regulation of cell-cycle progression and division
- PMID: 35491306
- PMCID: PMC9378598
- DOI: 10.1016/j.tcb.2022.03.010
Mechanical regulation of cell-cycle progression and division
Abstract
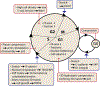
Cell-cycle progression and division are fundamental biological processes in animal cells, and their biochemical regulation has been extensively studied. An emerging body of work has revealed how mechanical interactions of cells with their microenvironment in tissues, including with the extracellular matrix (ECM) and neighboring cells, also plays a crucial role in regulating cell-cycle progression and division. We review recent work on how cells interpret physical cues and alter their mechanics to promote cell-cycle progression and initiate cell division, and then on how dividing cells generate forces on their surrounding microenvironment to successfully divide. Finally, the article ends by discussing how force generation during division potentially contributes to larger tissue-scale processes involved in development and homeostasis.
Keywords: cell cycle; cell division; extracellular matrix; force generation; mechanotransduction; microenvironment; mitosis.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no conflicts of interest.
Figures


References
-
- Morgan DO (2007) Cell Cycle: Principles of Control, New Science Press.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

