A novel superfamily of bridge-like lipid transfer proteins
- PMID: 35491307
- PMCID: PMC9588498
- DOI: 10.1016/j.tcb.2022.03.011
A novel superfamily of bridge-like lipid transfer proteins
Abstract
Lipid transfer proteins mediate nonvesicular transport of lipids at membrane contact sites to regulate the lipid composition of organelle membranes. Recently, a new type of bridge-like lipid transfer protein has emerged; these proteins contain a long hydrophobic groove and can mediate bulk transport of lipids between organelles. Here, we review recent insights into the structure of these proteins and identify a repeating modular unit that we propose to name the repeating β-groove (RBG) domain. This new structural understanding conceptually unifies all the RBG domain-containing lipid transfer proteins as members of an RBG protein superfamily. We also examine the biological functions of these lipid transporters in normal physiology and disease and speculate on the evolutionary origins of RBG proteins in bacteria.
Keywords: AlphaFold; Vps13; autophagy; lipid transfer proteins; lipids; membrane contact sites.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
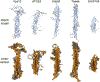


References
-
- DeGrella RF and Simoni RD (1982) Intracellular transport of cholesterol to the plasma membrane. J. Biol. Chem 257, 14256–14262 - PubMed
-
- Wirtz KW and Zilversmit DB (1968) Exchange of phospholipids between liver mitochondria and microsomes in vitro. J. Biol. Chem 243, 3596–3602 - PubMed
-
- Wong LH et al. (2019) Lipid transfer proteins: the lipid commute via shuttles, bridges and tubes. Nat. Rev. Mol. Cell Biol 20, 85–101 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

