Exercise Causes Arrhythmogenic Remodeling of Intracellular Calcium Dynamics in Plakophilin-2-Deficient Hearts
- PMID: 35491884
- PMCID: PMC9086182
- DOI: 10.1161/CIRCULATIONAHA.121.057757
Exercise Causes Arrhythmogenic Remodeling of Intracellular Calcium Dynamics in Plakophilin-2-Deficient Hearts
Abstract
Background: Exercise training, and catecholaminergic stimulation, increase the incidence of arrhythmic events in patients affected with arrhythmogenic right ventricular cardiomyopathy correlated with plakophilin-2 (PKP2) mutations. Separate data show that reduced abundance of PKP2 leads to dysregulation of intracellular Ca2+ (Ca2+i) homeostasis. Here, we study the relation between excercise, catecholaminergic stimulation, Ca2+i homeostasis, and arrhythmogenesis in PKP2-deficient murine hearts.
Methods: Experiments were performed in myocytes from a cardiomyocyte-specific, tamoxifen-activated, PKP2 knockout murine line (PKP2cKO). For training, mice underwent 75 minutes of treadmill running once per day, 5 days each week for 6 weeks. We used multiple approaches including imaging, high-resolution mass spectrometry, electrocardiography, and pharmacological challenges to study the functional properties of cells/hearts in vitro and in vivo.
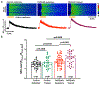
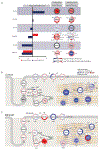
Results: In myocytes from PKP2cKO animals, training increased sarcoplasmic reticulum Ca2+ load, increased the frequency and amplitude of spontaneous ryanodine receptor (ryanodine receptor 2)-mediated Ca2+ release events (sparks), and changed the time course of sarcomeric shortening. Phosphoproteomics analysis revealed that training led to hyperphosphorylation of phospholamban in residues 16 and 17, suggesting a catecholaminergic component. Isoproterenol-induced increase in Ca2+i transient amplitude showed a differential response to β-adrenergic blockade that depended on the purported ability of the blockers to reach intracellular receptors. Additional experiments showed significant reduction of isoproterenol-induced Ca2+i sparks and ventricular arrhythmias in PKP2cKO hearts exposed to an experimental blocker of ryanodine receptor 2 channels.
Conclusions: Exercise disproportionately affects Ca2+i homeostasis in PKP2-deficient hearts in a manner facilitated by stimulation of intracellular β-adrenergic receptors and hyperphosphorylation of phospholamban. These cellular changes create a proarrhythmogenic state that can be mitigated by ryanodine receptor 2 blockade. Our data unveil an arrhythmogenic mechanism for exercise-induced or catecholaminergic life-threatening arrhythmias in the setting of PKP2 deficit. We suggest that membrane-permeable β-blockers are potentially more efficient for patients with arrhythmogenic right ventricular cardiomyopathy, highlight the potential for ryanodine receptor 2 channel blockers as treatment for the control of heart rhythm in the population at risk, and propose that PKP2-dependent and phospholamban-dependent arrhythmogenic right ventricular cardiomyopathy-related arrhythmias have a common mechanism.
Keywords: arrhythmogenic right ventricular cardiomyopathy; exercise; phospholamban; plakophilins; receptors, adrenergic, beta-1.
Conflict of interest statement
DISCLOSURES
Authors have no conflict of interests to disclose.
Figures


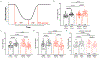



References
-
- Corrado D, Link MS, Calkins H. Arrhythmogenic Right Ventricular Cardiomyopathy. N Engl J Med. 2017;376:1489–1490. - PubMed
-
- Corrado D, Link MS, Calkins H. Arrhythmogenic Right Ventricular Cardiomyopathy. N Engl J Med. 2017;376:61–72. - PubMed
-
- Groeneweg JA, Bhonsale A, James CA, te Riele AS, Dooijes D, Tichnell C, Murray B, Wiesfeld ACP, Sawant AC, Kassamali B, et al. , Calkins H. Clinical Presentation, Long-Term Follow-Up, and Outcomes of 1001 Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy Patients and Family Members. Circ Cardiovasc Genet. 2015;8:437–446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

