Time-Frequency Representations of Brain Oscillations: Which One Is Better?
- PMID: 35492077
- PMCID: PMC9050353
- DOI: 10.3389/fninf.2022.871904
Time-Frequency Representations of Brain Oscillations: Which One Is Better?
Abstract
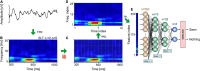
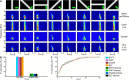
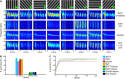
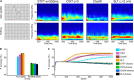
Brain oscillations are thought to subserve important functions by organizing the dynamical landscape of neural circuits. The expression of such oscillations in neural signals is usually evaluated using time-frequency representations (TFR), which resolve oscillatory processes in both time and frequency. While a vast number of methods exist to compute TFRs, there is often no objective criterion to decide which one is better. In feature-rich data, such as that recorded from the brain, sources of noise and unrelated processes abound and contaminate results. The impact of these distractor sources is especially problematic, such that TFRs that are more robust to contaminants are expected to provide more useful representations. In addition, the minutiae of the techniques themselves impart better or worse time and frequency resolutions, which also influence the usefulness of the TFRs. Here, we introduce a methodology to evaluate the "quality" of TFRs of neural signals by quantifying how much information they retain about the experimental condition during visual stimulation and recognition tasks, in mice and humans, respectively. We used machine learning to discriminate between various experimental conditions based on TFRs computed with different methods. We found that various methods provide more or less informative TFRs depending on the characteristics of the data. In general, however, more advanced techniques, such as the superlet transform, seem to provide better results for complex time-frequency landscapes, such as those extracted from electroencephalography signals. Finally, we introduce a method based on feature perturbation that is able to quantify how much time-frequency components contribute to the correct discrimination among experimental conditions. The methodology introduced in the present study may be extended to other analyses of neural data, enabling the discovery of data features that are modulated by the experimental manipulation.
Keywords: electroencephalography; explainable AI; machine learning; neural oscillations; neurophysiology; time-frequency representation.
Copyright © 2022 Bârzan, Ichim, Moca and Mureşan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Time Is of the Essence: Neural Codes, Synchronies, Oscillations, Architectures.Front Comput Neurosci. 2022 Jun 15;16:898829. doi: 10.3389/fncom.2022.898829. eCollection 2022. Front Comput Neurosci. 2022. PMID: 35814343 Free PMC article.
-
Sharp detection of oscillation packets in rich time-frequency representations of neural signals.Front Hum Neurosci. 2023 Dec 7;17:1112415. doi: 10.3389/fnhum.2023.1112415. eCollection 2023. Front Hum Neurosci. 2023. PMID: 38144896 Free PMC article.
-
Recording human electrocorticographic (ECoG) signals for neuroscientific research and real-time functional cortical mapping.J Vis Exp. 2012 Jun 26;(64):3993. doi: 10.3791/3993. J Vis Exp. 2012. PMID: 22782131 Free PMC article.
-
Neural Cross-Frequency Coupling: Connecting Architectures, Mechanisms, and Functions.Trends Neurosci. 2015 Nov;38(11):725-740. doi: 10.1016/j.tins.2015.09.001. Trends Neurosci. 2015. PMID: 26549886 Review.
-
Auditory representations for long lasting sounds: Insights from event-related brain potentials and neural oscillations.Brain Lang. 2023 Feb;237:105221. doi: 10.1016/j.bandl.2022.105221. Epub 2023 Jan 7. Brain Lang. 2023. PMID: 36623340 Review.
Cited by
-
Interpretable many-class decoding for MEG.Neuroimage. 2023 Nov 15;282:120396. doi: 10.1016/j.neuroimage.2023.120396. Epub 2023 Oct 5. Neuroimage. 2023. PMID: 37805019 Free PMC article.
-
Time Is of the Essence: Neural Codes, Synchronies, Oscillations, Architectures.Front Comput Neurosci. 2022 Jun 15;16:898829. doi: 10.3389/fncom.2022.898829. eCollection 2022. Front Comput Neurosci. 2022. PMID: 35814343 Free PMC article.
-
Neural oscillations in the ventral striatum reveal differences between the encoding of palatable food and ethanol consumption.Alcohol Clin Exp Res (Hoboken). 2023 Jul;47(7):1327-1340. doi: 10.1111/acer.15101. Epub 2023 Jun 4. Alcohol Clin Exp Res (Hoboken). 2023. PMID: 37166071 Free PMC article.
-
The gamma rhythm as a guardian of brain health.Elife. 2024 Nov 20;13:e100238. doi: 10.7554/eLife.100238. Elife. 2024. PMID: 39565646 Free PMC article. Review.
-
Chloride deregulation and GABA depolarization in MTOR-related malformations of cortical development.Brain. 2025 Feb 3;148(2):549-563. doi: 10.1093/brain/awae262. Brain. 2025. PMID: 39106285 Free PMC article.
References
-
- Barredo Arrieta A., Díaz-Rodríguez N., Del Ser J., Bennetot A., Tabik S., Barbado A., et al. (2020). Explainable Artificial intelligence (XAI): concepts, taxonomies, opportunities and challenges toward responsible AI. Inf. Fusion 58 82–115. 10.1016/j.inffus.2019.12.012 - DOI
-
- Bârzan H., Moca V. V., Ichim A.-M., Muresan R. C. (2021). “Fractional Superlets,” in Proceedings of the 2020 28th European Signal Processing Conference (EUSIPCO). Presented at the 2020 28th European Signal Processing Conference (EUSIPCO), (Amsterdam: EUSIPCO; ). 2220–2224. 10.23919/Eusipco47968.2020.9287873 - DOI
LinkOut - more resources
Full Text Sources

