Inhibitors of dihydroorotate dehydrogenase cooperate with molnupiravir and N4-hydroxycytidine to suppress SARS-CoV-2 replication
- PMID: 35492218
- PMCID: PMC9035612
- DOI: 10.1016/j.isci.2022.104293
Inhibitors of dihydroorotate dehydrogenase cooperate with molnupiravir and N4-hydroxycytidine to suppress SARS-CoV-2 replication
Abstract
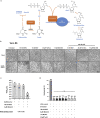
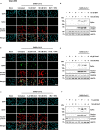
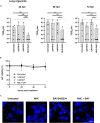
The nucleoside analog N4-hydroxycytidine (NHC) is the active metabolite of the prodrug molnupiravir, which has been approved for the treatment of COVID-19. SARS-CoV-2 incorporates NHC into its RNA, resulting in defective virus genomes. Likewise, inhibitors of dihydroorotate dehydrogenase (DHODH) reduce virus yield upon infection, by suppressing the cellular synthesis of pyrimidines. Here, we show that NHC and DHODH inhibitors strongly synergize in the inhibition of SARS-CoV-2 replication in vitro. We propose that the lack of available pyrimidine nucleotides upon DHODH inhibition increases the incorporation of NHC into nascent viral RNA. This concept is supported by the rescue of virus replication upon addition of pyrimidine nucleosides to the media. DHODH inhibitors increased the antiviral efficiency of molnupiravir not only in organoids of human lung, but also in Syrian Gold hamsters and in K18-hACE2 mice. Combining molnupiravir with DHODH inhibitors may thus improve available therapy options for COVID-19.
Keywords: Drugs; Virology.
© 2022 The Author(s).
Conflict of interest statement
AS, HK, EP, and DV are employees of Immunic AG and own shares and/or stock-options of the parent company of Immunic AG, Immunic Inc. Some of the Immunic AG employees also hold patents for the Immunic compounds described in this manuscript (WO2012/001,148, WO03006425). KMS, AD, and MD are employees of University Medical Center Göttingen, which has signed a License Agreement with Immunic AG covering the combination of DHODH inhibitors and nucleoside analogs to treat viral infections, including COVID-19 (inventors: MD, KMS, and AD). The other authors declare no conflict of interest.
Figures






References
-
- Agostini M.L., Pruijssers A.J., Chappell J.D., Gribble J., Lu X., Andres E.L., Bluemling G.R., Lockwood M.A., Sheahan T.P., Sims A.C., et al. Small-molecule antiviral β-d-N (4)-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance. J. Virol. 2019;93:e01348-19. doi: 10.1128/jvi.01348-19. - DOI - PMC - PubMed
-
- Barnard D.L., Hubbard V.D., Burton J., Smee D.F., Morrey J.D., Otto M.J., Sidwell R.W. Inhibition of severe acute respiratory syndrome-associated coronavirus (SARSCoV) by calpain inhibitors and beta-D-N4-hydroxycytidine. Antivir. Chem. Chemother. 2004;15:15–22. doi: 10.1177/095632020401500102. - DOI - PubMed
-
- Bliss C.I. The toxicity of poisons applied jointly. Ann. Appl. Biol. 1939;26:585–615. doi: 10.1111/j.1744-7348.1939.tb06990.x. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

