Crystal structure and Hirshfeld surface analysis of (E)-1-[2,2-di-bromo-1-(2-nitro-phen-yl)ethen-yl]-2-(4-fluoro-phen-yl)diazene
- PMID: 35492284
- PMCID: PMC8983971
- DOI: 10.1107/S205698902200278X
Crystal structure and Hirshfeld surface analysis of (E)-1-[2,2-di-bromo-1-(2-nitro-phen-yl)ethen-yl]-2-(4-fluoro-phen-yl)diazene
Abstract
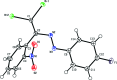
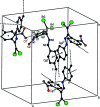
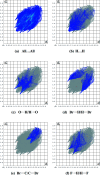
In the title compound, C14H8Br2FN3O2, the nitro-substituted benzene ring and the 4-fluoro-phenyl ring form a dihedral angle of 65.73 (7)°. In the crystal, mol-ecules are linked into chains by C-H⋯O hydrogen bonds running parallel to the c-axis direction. The crystal packing is consolidated by C-F⋯π inter-actions and π-π stacking inter-actions, and short Br⋯O [2.9828 (13) Å] contacts are observed. The Hirshfeld surface analysis of the crystal structure indicates that the most important contributions to the crystal packing are from H⋯H (17.4%), O⋯H/H⋯O (16.3%), Br⋯H/H⋯Br (15.5%), Br⋯C/C⋯Br (10.1%) and F⋯H/H⋯F (8.1%) contacts.
Keywords: C—F⋯π interaction; Hirshfeld surface analysis; crystal structure; π–π stacking interaction.
© Çelikesir et al. 2022.
Figures

References
-
- Bruker (2018). APEX3 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
