Drying banana seeds for ex situ conservation
- PMID: 35492425
- PMCID: PMC9041424
- DOI: 10.1093/conphys/coab099
Drying banana seeds for ex situ conservation
Erratum in
-
Corrigendum to: Drying banana seeds for ex situ conservation.Conserv Physiol. 2022 Jan 26;10(1):coac005. doi: 10.1093/conphys/coac005. eCollection 2022 Jan 1. Conserv Physiol. 2022. PMID: 35100328 Free PMC article.
Abstract
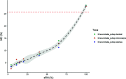
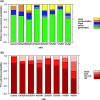
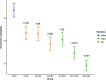
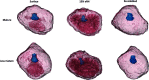
The ability of seeds to withstand drying is fundamental to ex situ seed conservation but drying responses are not well known for most wild species including crop wild relatives. We look at drying responses of seeds of Musa acuminata and Musa balbisiana, the two primary wild relatives of bananas and plantains, using the following four experimental approaches: (i) We equilibrated seeds to a range of relative humidity (RH) levels using non-saturated lithium chloride solutions and subsequently measured moisture content (MC) and viability. At each humidity level we tested viability using embryo rescue (ER), tetrazolium chloride staining and germination in an incubator. We found that seed viability was not reduced when seeds were dried to 4% equilibrium relative humidity (eRH; equating to 2.5% MC). (ii) We assessed viability of mature and less mature seeds using ER and germination in the soil and tested responses to drying. Findings showed that seeds must be fully mature to germinate and immature seeds had negligible viability. (iii) We dried seeds extracted from ripe/unripe fruit to 35-40% eRH at different rates and tested viability with germination tests in the soil. Seeds from unripe fruit lost viability when dried and especially when dried faster; seeds from ripe fruit only lost viability when fast dried. (iv) Finally, we dried and re-imbibed mature and less mature seeds and measured embryo shrinkage and volume change using X-ray computer tomography. Embryos of less mature seeds shrank significantly when dried to 15% eRH from 0.468 to 0.262 mm3, but embryos of mature seeds did not. Based on our results, mature seeds from ripe fruit are desiccation tolerant to moisture levels required for seed genebanking but embryos from immature seeds are mechanistically less able to withstand desiccation, especially when water potential gradients are high.
Keywords: Crop wild relatives; desiccation tolerance; genebank; genetic resources.
© The Author(s) 2022. Published by Oxford University Press and the Society for Experimental Biology.
Figures



Similar articles
-
Challenges for Ex Situ Conservation of Wild Bananas: Seeds Collected in Papua New Guinea Have Variable Levels of Desiccation Tolerance.Plants (Basel). 2020 Sep 21;9(9):1243. doi: 10.3390/plants9091243. Plants (Basel). 2020. PMID: 32967145 Free PMC article.
-
Maximizing genetic representation in seed collections from populations of self and cross-pollinated banana wild relatives.BMC Plant Biol. 2021 Sep 9;21(1):415. doi: 10.1186/s12870-021-03142-y. BMC Plant Biol. 2021. PMID: 34503446 Free PMC article.
-
Increases in the longevity of desiccation-phase developing rice seeds: response to high-temperature drying depends on harvest moisture content.Ann Bot. 2015 Aug;116(2):247-59. doi: 10.1093/aob/mcv091. Epub 2015 Jul 1. Ann Bot. 2015. PMID: 26133688 Free PMC article.
-
Pollen and seed desiccation tolerance in relation to degree of developmental arrest, dispersal, and survival.J Exp Bot. 2011 Nov;62(15):5267-81. doi: 10.1093/jxb/err154. Epub 2011 Aug 9. J Exp Bot. 2011. PMID: 21831844 Review.
-
Seed Moisture Isotherms, Sorption Models, and Longevity.Front Plant Sci. 2022 Jun 2;13:891913. doi: 10.3389/fpls.2022.891913. eCollection 2022. Front Plant Sci. 2022. PMID: 35720538 Free PMC article.
Cited by
-
The a-peel of storing mature seeds for wild banana conservation.Conserv Physiol. 2022 Jul 22;10(1):coac049. doi: 10.1093/conphys/coac049. eCollection 2022. Conserv Physiol. 2022. PMID: 35875678 Free PMC article. No abstract available.
References
-
- Abdelnouresquivel A, Mora A, Villalobos V (1992) Cryopreservation of zygotic embryos of Musa acuminata (AA) and M. balbisiana (BB). Cryo Lett 13: 159–164.
-
- Ballesteros D, Pritchard HW, Walters C (2020) Dry architecture: towards the understanding of the variation of longevity in desiccation-tolerant germplasm. Seed Sci Res 30: 142–155.
-
- Bohra P, Waman AA, Jerard BA (2020) Seed germination and storage studies in seed-fertile Musa indandamanensis and its conservation. S Afr J Bot 128: 161–166.
LinkOut - more resources
Full Text Sources

