Separation of neodymium and dysprosium by solvent extraction using ionic liquids combined with neutral extractants: batch and mixer-settler experiments
- PMID: 35492521
- PMCID: PMC9047729
- DOI: 10.1039/c9ra08996a
Separation of neodymium and dysprosium by solvent extraction using ionic liquids combined with neutral extractants: batch and mixer-settler experiments
Abstract
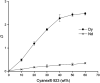
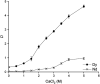
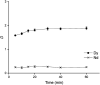
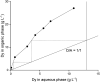
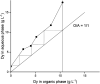
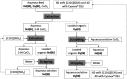
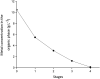
A solvent extraction method based on the combination of the ionic liquid trihexyl(tetradecyl)phosphonium thiocyanate or nitrate ([C101][SCN], [C101][NO3]) and the neutral extractants Cyanex 923 or tri-n-butyl phosphate (TBP) has been investigated for the separation of Nd(iii) and Dy(iii) from chloride media. High distribution ratios and separation factors were obtained when using Cyanex 923 diluted in [C101][SCN] 40 : 60 (wt%) and extracting from chloride media. The addition of Cyanex 923 to the ionic liquid has four advantages: (1) increase in the distribution ratios of the rare earths, (2) decrease of the viscosity of the organic phase, hence an improved mass transfer, (3) increase in the loading capacity of the ionic liquid and (4) improvement of the coalescence and phase disengagement, which is of importance when carrying out separations in continuous mode. Different extraction parameters were optimized: concentration of Cyanex 923, chloride concentration in the aqueous phase, equilibration time, pH of the aqueous phase, type of scrubbing and stripping agents. The ionic liquid combined with Cyanex 923 was recycled up to three times without losing its extraction efficiency. McCabe-Thiele diagrams were constructed to determine the number of stages needed for the separation of Nd(iii) and Dy(iii). Stripping of Dy(iii) from the organic phase was easily achieved with water. The feasibility to run this process in continuous mode was tested in a battery of small mixer-settlers (0.12 L and 0.48 L effective volume in the mixer and the settler, respectively). As a result, this process constitutes a novel and scalable alternative for the separation of Nd(iii) and Dy(iii).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Golev A. Scott M. Erskine P. D. Ali S. H. Ballantyne G. R. Resour. Policy. 2014;41:52–59. doi: 10.1016/j.resourpol.2014.03.004. - DOI
-
- Binnemans K. Jones P. T. Blanpain B. Van Gerven T. Yang Y. Walton A. Buchert M. J. Cleaner Prod. 2013;51:1–22. doi: 10.1016/j.jclepro.2012.12.037. - DOI
-
- Yang Y. Walton A. Sheridan R. Güth K. Gauß R. Gutfleisch O. Buchert M. Steenari B.-M. Van Gerven T. Jones P. T. Binnemans K. J. Sustain. Metall. 2017;3:122–149. doi: 10.1007/s40831-016-0090-4. - DOI
-
- Brown D. Ma B. M. Chen Z. J. Magn. Magn. Mater. 2002;248:432–440. doi: 10.1016/S0304-8853(02)00334-7. - DOI
-
- Zepf V., Rare earth elements a new approach to the nexus of supply, demand and use: exemplified along the use of neodymium in permanent, Augsburg University, 2013
LinkOut - more resources
Full Text Sources
Research Materials

