A novel drug-drug coamorphous system without molecular interactions: improve the physicochemical properties of tadalafil and repaglinide
- PMID: 35492562
- PMCID: PMC9048229
- DOI: 10.1039/c9ra07149k
A novel drug-drug coamorphous system without molecular interactions: improve the physicochemical properties of tadalafil and repaglinide
Abstract
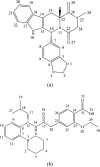
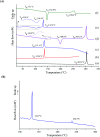
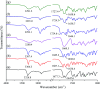
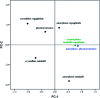
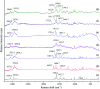
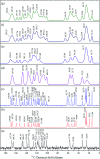
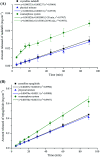
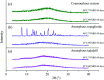
Tadalafil and repaglinide, categorized as BCS class II drugs, have low oral bioavailabilities due to their poorly aqueous solubilities and dissolutions. The aim of this study was to enhance the dissolution of tadalafil and repaglinide by co-amorphization technology and evaluate the storage and compression stability of such coamorphous system. Based on Flory-Huggins interaction parameter (χ ≤ 0) and Hansen solubility parameter (δ t ≤ 7 MPa0.5) calculations, tadalafil and repaglinide was predicted to be well miscible with each other. Coamorphous tadalafil-repaglinide (molar ratio, 1 : 1) was prepared by solvent-evaporation method and characterized with respect to its thermal properties, possible molecular interactions. A single T g (73.1 °C) observed in DSC and disappearance of crystallinity in PXRD indicated the formation of coamorphous system. Principal component analysis of FTIR in combination with Raman spectroscopy and Ss 13C NMR suggested the absence of intermolecular interactions in coamorphous tadalafil-repaglinide. In comparison to pure crystalline forms and their physical mixtures, both drugs in coamorphous system exhibited significant increases in intrinsic dissolution rate (1.5-3-fold) and could maintain supersaturated level for at least 4 hours in non-sink dissolution. In addition, the coamorphous tadalafil-repaglinide showed improved stability compared to the pure amorphous forms under long-term stability and accelerated storage conditions as well as under high compressing pressure. In conclusion, this study showed that co-amorphization technique is a promising approach for improving the dissolution rate of poorly water-soluble drugs and for stabilizing amorphous drugs.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Coamorphous System of Florfenicol-Oxymatrine for Improving the Solubility and Dissolution Rate of Florfenicol: Preparation, Characterization and Molecular Dynamics Simulation.J Pharm Sci. 2021 Jun;110(6):2544-2554. doi: 10.1016/j.xphs.2021.02.005. Epub 2021 Feb 9. J Pharm Sci. 2021. PMID: 33577826
-
Coamorphization combined with complexation enhances dissolution of lurasidone hydrochloride and puerarin with synchronized release.Int J Pharm. 2020 Oct 15;588:119793. doi: 10.1016/j.ijpharm.2020.119793. Epub 2020 Aug 19. Int J Pharm. 2020. PMID: 32827676
-
Coamorphous repaglinide-saccharin with enhanced dissolution.Int J Pharm. 2013 Jun 25;450(1-2):290-5. doi: 10.1016/j.ijpharm.2013.04.032. Epub 2013 Apr 21. Int J Pharm. 2013. PMID: 23612357
-
[Construction of Coamorphous Technology Aimed at Improving Membrane Permeability and Application to Emulsion].Yakugaku Zasshi. 2021;141(11):1223-1228. doi: 10.1248/yakushi.21-00137. Yakugaku Zasshi. 2021. PMID: 34719541 Review. Japanese.
-
Recent Advances in Enhancement of Dissolution and Supersaturation of Poorly Water-Soluble Drug in Amorphous Pharmaceutical Solids: A Review.AAPS PharmSciTech. 2021 Dec 10;23(1):16. doi: 10.1208/s12249-021-02137-0. AAPS PharmSciTech. 2021. PMID: 34893936 Review.
Cited by
-
Ball-Milling Preparation of the Drug-Drug Solid Form of Pioglitazone-Rosuvastatin at Different Molar Ratios: Characterization and Intrinsic Dissolution Rates Evaluation.Pharmaceutics. 2023 Feb 13;15(2):630. doi: 10.3390/pharmaceutics15020630. Pharmaceutics. 2023. PMID: 36839951 Free PMC article.
-
Application of Box-Behnken Design in the Preparation, Optimization, and In-Vivo Pharmacokinetic Evaluation of Oral Tadalafil-Loaded Niosomal Film.Pharmaceutics. 2023 Jan 3;15(1):173. doi: 10.3390/pharmaceutics15010173. Pharmaceutics. 2023. PMID: 36678802 Free PMC article.
-
Levofloxacin Cocrystal/Salt with Phthalimide and Caffeic Acid as Promising Solid-State Approach to Improve Antimicrobial Efficiency.Antibiotics (Basel). 2022 Jun 13;11(6):797. doi: 10.3390/antibiotics11060797. Antibiotics (Basel). 2022. PMID: 35740203 Free PMC article.
-
Development of spray-dried amorphous solid dispersions of tadalafil using glycyrrhizin for enhanced dissolution and aphrodisiac activity in male rats.Saudi Pharm J. 2020 Dec;28(12):1817-1826. doi: 10.1016/j.jsps.2020.11.007. Epub 2020 Nov 21. Saudi Pharm J. 2020. PMID: 33424269 Free PMC article.
-
Recent Advances in Co-Former Screening and Formation Prediction of Multicomponent Solid Forms of Low Molecular Weight Drugs.Pharmaceutics. 2023 Aug 22;15(9):2174. doi: 10.3390/pharmaceutics15092174. Pharmaceutics. 2023. PMID: 37765145 Free PMC article. Review.
References
-
- Aaltonen J. Rades T. Dissolution Technol. 2009;16:47–54. doi: 10.14227/DT160209P47. - DOI
LinkOut - more resources
Full Text Sources

