Prenatal GABAB Receptor Agonist Administration Corrects the Inheritance of Autism-Like Core Behaviors in Offspring of Mice Prenatally Exposed to Valproic Acid
- PMID: 35492716
- PMCID: PMC9051083
- DOI: 10.3389/fpsyt.2022.835993
Prenatal GABAB Receptor Agonist Administration Corrects the Inheritance of Autism-Like Core Behaviors in Offspring of Mice Prenatally Exposed to Valproic Acid
Abstract
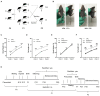
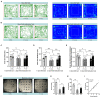
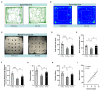
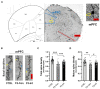
This study was performed to evaluate the effects of prenatal baclofen (a GABAB receptor agonist) treatment on the inheritance of autism-like behaviors in valproic acid (VPA)-exposed mice. VPA model mice (first generation, F1) that were prenatally exposed to VPA exhibited robust core autism-like behaviors, and we found that oral administration of baclofen to F1 mice corrected their autism-like behavioral phenotypes at an early age. Based on a previous epigenetics study, we mated the F1 male offspring with litter females to produce the second generation (F2). The F2 male mice showed obvious inheritance of autism-like phenotypes from F1 mice, implying the heritability of autism symptoms in patients with prenatal VPA exposure. Furthermore, we found prenatal baclofen administration was associated with beneficial effects on the autism-like phenotype in F2 male mice. This may have involved corrections in the density of total/mature dendritic spines in the hippocampus (HC) and medial prefrontal cortex (mPFC), normalizing synaptic plasticity. In this research, GABAB receptor agonist administration corrected the core autism-like behaviors of F1 mice and protected against the inheritance of neurodevelopmental disorders in the offspring of F1 mice, suggesting the potential of early intervention with GABAB receptor agonists in the treatment of neurodevelopmental disorders.
Keywords: GABAB; autism spectrum disorder (ASD); baclofen; inheritance; mice; valproic acid (VPA).
Copyright © 2022 Jiang, He, Xiao, Sun, Ding, Li, Guo, Wang, Wang, Gao, Sun and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
The GABAB receptor agonist STX209 reverses the autism‑like behaviour in an animal model of autism induced by prenatal exposure to valproic acid.Mol Med Rep. 2022 May;25(5):154. doi: 10.3892/mmr.2022.12670. Epub 2022 Mar 4. Mol Med Rep. 2022. PMID: 35244195 Free PMC article.
-
The transgenerational inheritance of autism-like phenotypes in mice exposed to valproic acid during pregnancy.Sci Rep. 2016 Nov 7;6:36250. doi: 10.1038/srep36250. Sci Rep. 2016. PMID: 27819277 Free PMC article.
-
[Mechanism of valproic acid-induced dendritic spine and synaptic impairment in the prefrontal cortex for causing core autistic symptoms in mice].Nan Fang Yi Ke Da Xue Xue Bao. 2022 Jan 20;42(1):101-107. doi: 10.12122/j.issn.1673-4254.2022.01.12. Nan Fang Yi Ke Da Xue Xue Bao. 2022. PMID: 35249876 Free PMC article. Chinese.
-
Risperidone and aripiprazole alleviate prenatal valproic acid-induced abnormalities in behaviors and dendritic spine density in mice.Psychopharmacology (Berl). 2017 Nov;234(21):3217-3228. doi: 10.1007/s00213-017-4703-9. Epub 2017 Aug 10. Psychopharmacology (Berl). 2017. PMID: 28798977
-
Prenatal valproate in rodents as a tool to understand the neural underpinnings of social dysfunctions in autism spectrum disorder.Neuropharmacology. 2019 Nov 15;159:107477. doi: 10.1016/j.neuropharm.2018.12.024. Epub 2019 Jan 9. Neuropharmacology. 2019. PMID: 30639388 Review.
Cited by
-
Generational synaptic functions of GABAA receptor β3 subunit deteriorations in an animal model of social deficit.J Biomed Sci. 2022 Jul 11;29(1):51. doi: 10.1186/s12929-022-00835-w. J Biomed Sci. 2022. PMID: 35821032 Free PMC article.
-
Benefits of bone marrow mesenchymal stem cells compared to their conditioned medium in valproic acid-induced autism in rats.Mol Biol Rep. 2024 Feb 24;51(1):353. doi: 10.1007/s11033-024-09292-0. Mol Biol Rep. 2024. PMID: 38401030
-
Autism Spectrum Disorder: Neurodevelopmental Risk Factors, Biological Mechanism, and Precision Therapy.Int J Mol Sci. 2023 Jan 17;24(3):1819. doi: 10.3390/ijms24031819. Int J Mol Sci. 2023. PMID: 36768153 Free PMC article. Review.
-
GABAB Receptor: Structure, Biological Functions, and Therapy for Diseases.MedComm (2020). 2025 Apr 16;6(5):e70163. doi: 10.1002/mco2.70163. eCollection 2025 May. MedComm (2020). 2025. PMID: 40242161 Free PMC article. Review.
References
-
- Christensen Deborah L, Maenner Matthew J, Deborah B, Constantino John N, Julie D, Durkin Maureen S, et al. . Prevalence and characteristics of autism spectrum disorder among children aged 4 years - early autism and developmental disabilities monitoring network, seven sites, United States, 2010, 2012, and 2014. MMWR Surveill Summ. (2019) 68:1–19. 10.15585/mmwr.ss6802a1 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

