Dopamine Supersensitivity: A Novel Hypothesis of Opioid-Induced Neurobiological Mechanisms Underlying Opioid-Stimulant Co-use and Opioid Relapse
- PMID: 35492733
- PMCID: PMC9051080
- DOI: 10.3389/fpsyt.2022.835816
Dopamine Supersensitivity: A Novel Hypothesis of Opioid-Induced Neurobiological Mechanisms Underlying Opioid-Stimulant Co-use and Opioid Relapse
Abstract
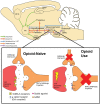
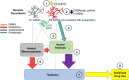
Emergent harms presented by the co-use of opioids and methamphetamine highlight the broader public health challenge of preventing and treating opioid and stimulant co-use. Development of effective therapeutics requires an understanding of the physiological mechanisms that may be driving co-use patterns, specifically the underlying neurobiology of co-use and how they may facilitate (or be leveraged to prevent) continued use patterns. This narrative review summarizes largely preclinical data that demonstrate clinically-meaningful relationships between the dopamine and opioid systems with direct implications for opioid and stimulant co-use. Synthesized conclusions of this body of research include evidence that changes in the dopamine system occur only once physical dependence to opioids develops, that the chronicity of opioid exposure is associated with the severity of changes, and that withdrawal leaves the organism in a state of substantive dopamine deficit that persists long after the somatic or observed signs of opioid withdrawal appear to have resolved. Evidence also suggests that dopamine supersensitivity develops soon after opioid abstinence and results in increased response to dopamine agonists that increases in magnitude as the abstinence period continues and is evident several weeks into protracted withdrawal. Mechanistically, this supersensitivity appears to be mediated by changes in the sensitivity, not quantity, of dopamine D2 receptors. Here we propose a neural circuit mechanism unique to withdrawal from opioid use with implications for increased stimulant sensitivity in previously stimulant-naïve or inexperienced populations. These hypothesized effects collectively delineate a mechanism by which stimulants would be uniquely reinforcing to persons with opioid physical dependence, would contribute to the acute opioid withdrawal syndrome, and could manifest subjectively as craving and/or motivation to use that could prompt opioid relapse during acute and protracted withdrawal. Preclinical research is needed to directly test these hypothesized mechanisms. Human laboratory and clinical trial research is needed to explore these clinical predictions and to advance the goal of developing treatments for opioid-stimulant co-use and/or opioid relapse prevention and withdrawal remediation.
Keywords: cocaine; methamphetamine; opioid; relapse; stimulant; treatment; withdrawal.
Copyright © 2022 Strickland, Gipson and Dunn.
Conflict of interest statement
In the past 3 years, KD has consulted for Canopy Corporation, Beckley-Canopy, and MindMed. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Dopamine D1 receptor antagonist reduces stimulant-induced conditioned place preferences and dopamine receptor supersensitivity.Naunyn Schmiedebergs Arch Pharmacol. 2020 Jan;393(1):131-138. doi: 10.1007/s00210-019-01694-3. Epub 2019 Aug 1. Naunyn Schmiedebergs Arch Pharmacol. 2020. PMID: 31372696
-
Impact of Morphine Dependence and Withdrawal on the Reinforcing Effectiveness of Fentanyl, Cocaine, and Methamphetamine in Rats.Front Pharmacol. 2021 May 21;12:691700. doi: 10.3389/fphar.2021.691700. eCollection 2021. Front Pharmacol. 2021. PMID: 34093214 Free PMC article.
-
Bouncing back: Brain rehabilitation amid opioid and stimulant epidemics.Neuroimage Clin. 2019;24:102068. doi: 10.1016/j.nicl.2019.102068. Epub 2019 Nov 5. Neuroimage Clin. 2019. PMID: 31795056 Free PMC article. Review.
-
Methamphetamine abstinence induces changes in μ-opioid receptor, oxytocin and CRF systems: Association with an anxiogenic phenotype.Neuropharmacology. 2016 Jun;105:520-532. doi: 10.1016/j.neuropharm.2016.02.012. Epub 2016 Feb 16. Neuropharmacology. 2016. PMID: 26896754
-
Modulation of drug choice by extended drug access and withdrawal in rhesus monkeys: Implications for negative reinforcement as a driver of addiction and target for medications development.Pharmacol Biochem Behav. 2018 Jan;164:32-39. doi: 10.1016/j.pbb.2017.04.006. Epub 2017 Apr 22. Pharmacol Biochem Behav. 2018. PMID: 28442370 Free PMC article. Review.
Cited by
-
Oxycodone withdrawal is associated with increased cocaine self-administration and aberrant accumbens glutamate plasticity in rats.Neuropharmacology. 2024 Jan 1;242:109773. doi: 10.1016/j.neuropharm.2023.109773. Epub 2023 Oct 21. Neuropharmacology. 2024. PMID: 37865136 Free PMC article.
-
Clinical management of psychostimulant withdrawal: review of the evidence.Addiction. 2023 Apr;118(4):750-762. doi: 10.1111/add.16093. Epub 2022 Dec 12. Addiction. 2023. PMID: 36401591 Free PMC article. Review.
-
Animal models of cocaine use: importance of social context and co-use.Trends Pharmacol Sci. 2025 Mar;46(3):220-230. doi: 10.1016/j.tips.2025.01.003. Epub 2025 Jan 28. Trends Pharmacol Sci. 2025. PMID: 39875301 Review.
-
Exploring Nepicastat Activity: Beyond DβH.Int J Mol Sci. 2025 May 3;26(9):4356. doi: 10.3390/ijms26094356. Int J Mol Sci. 2025. PMID: 40362592 Free PMC article.
-
Integrative multi-dimensional characterization of striatal projection neuron heterogeneity in adult brain.bioRxiv [Preprint]. 2023 Oct 21:2023.05.04.539488. doi: 10.1101/2023.05.04.539488. bioRxiv. 2023. PMID: 37205475 Free PMC article. Preprint.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

