La(OH)3 nanoparticles immobilized on Fe3O4@chitosan composites as novel magnetic nanocatalysts for sonochemical oxidation of benzyl alcohol to benzaldehyde
- PMID: 35492745
- PMCID: PMC9043185
- DOI: 10.1039/d1ra05848g
La(OH)3 nanoparticles immobilized on Fe3O4@chitosan composites as novel magnetic nanocatalysts for sonochemical oxidation of benzyl alcohol to benzaldehyde
Abstract
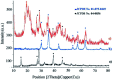
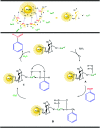
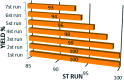
This work introduces an eco-friendly method for immobilization of La(OH)3 nanoparticles on modified Fe3O4 nanoparticles. The structural and morphological characteristics of the nanocatalyst were determined by various analytical techniques including, FT-IR, EDS, FESEM, VSM and XRD. The catalytic efficiency of the Fe3O4@Cs/La(OH)3 composite as a heterogeneous nanocatalyst was evaluated by selective oxidation of benzylic alcohols to aldehydes. The optimum reaction conditions including time, temperature, nanocatalyst dosage, and solvent were investigated for ultrasound-assisted oxidation processes. Furthermore, the magnetic nanocatalyst was recovered up to seven times without considerable activity loss. Furthermore, the proposed nanocomposite had a remarkable effect on reducing the reaction time and enhancing the yield.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors stated that they had no financial or personal interest in preparing the material reported in this article.
Figures






References
LinkOut - more resources
Full Text Sources

