Chemistry and biological activities of hetisine-type diterpenoid alkaloids
- PMID: 35492752
- PMCID: PMC9043348
- DOI: 10.1039/d1ra07173d
Chemistry and biological activities of hetisine-type diterpenoid alkaloids
Abstract
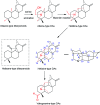
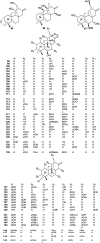
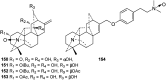
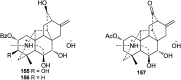
Hetisine-type C20-diterpenoid alkaloids (DAs) are one of the most important DA subtypes. During the past decades, a total of 157 hetisine-type DAs were obtained from plants from seven genera in three families, most of which were isolated from the genera Aconitum and Delphinium in the Ranunculaceae family. Structurally, hetisine-type DAs are characterized by a heptacyclic hetisane skeleton formed by the linkage of C(14)-C(20) and N-C(6) bonds in an atisine-type DA, and their structural diversity is created by the states of the N atom and various substituents. Pharmacological studies have revealed a wide range of pharmacological actions for hetisine-type DAs, including antiarrhythmic, antitumor, antimicrobial and insecticidal activities, as well as effects on peripheral vasculature, which are closely related to their chemical structures. In particular, the prominent antiarrhythmic effects and low toxicity of hetisine-type DAs highlight their potential in antiarrhythmic drug discovery. Hetisine-type DAs with diverse bioactivities are promising lead structures for further development as commercial agents in medicine.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Overview of the chemistry and biological activities of natural atisine-type diterpenoid alkaloids.RSC Adv. 2024 Jul 22;14(32):22882-22893. doi: 10.1039/d4ra03305a. eCollection 2024 Jul 19. RSC Adv. 2024. PMID: 39040692 Free PMC article. Review.
-
C18-diterpenoid alkaloids in tribe Delphineae (Ranunculaceae): phytochemistry, chemotaxonomy, and bioactivities.RSC Adv. 2021 Dec 22;12(1):395-405. doi: 10.1039/d1ra08132b. eCollection 2021 Dec 20. RSC Adv. 2021. PMID: 35424499 Free PMC article. Review.
-
Natural Kaurane-class of Diterpenoid Alkaloids: Structure, Distribution, and Bioactivities.Chem Biodivers. 2025 Jun;22(6):e202403271. doi: 10.1002/cbdv.202403271. Epub 2025 Feb 1. Chem Biodivers. 2025. PMID: 39814687 Review.
-
Anti-inflammatory diterpenoid alkaloids from Aconitum tanguticum (Maxim.) Stapf.Phytochemistry. 2023 Feb;206:113524. doi: 10.1016/j.phytochem.2022.113524. Epub 2022 Dec 1. Phytochemistry. 2023. PMID: 36464099
-
Enantioselective approach to the hetisine alkaloids. Synthesis of the 3-methyl-1-aza-tricyclo[5.2.1.0(3,8)]decane core via intramolecular dipolar cycloaddition.Org Lett. 2005 Jul 21;7(15):3323-5. doi: 10.1021/ol051184v. Org Lett. 2005. PMID: 16018651 Free PMC article.
Cited by
-
The Chemical Ecology of Plant Natural Products.Prog Chem Org Nat Prod. 2024;124:57-183. doi: 10.1007/978-3-031-59567-7_2. Prog Chem Org Nat Prod. 2024. PMID: 39101984 Review.
-
Exploring the Biomedical Potential of Terpenoid Alkaloids: Sources, Structures, and Activities.Molecules. 2024 Apr 25;29(9):1968. doi: 10.3390/molecules29091968. Molecules. 2024. PMID: 38731459 Free PMC article. Review.
-
Overview of the chemistry and biological activities of natural atisine-type diterpenoid alkaloids.RSC Adv. 2024 Jul 22;14(32):22882-22893. doi: 10.1039/d4ra03305a. eCollection 2024 Jul 19. RSC Adv. 2024. PMID: 39040692 Free PMC article. Review.
-
Finding activity through rigidity: syntheses of natural products containing tricyclic bridgehead carbon centers.Nat Prod Rep. 2023 Aug 16;40(8):1393-1431. doi: 10.1039/d3np00008g. Nat Prod Rep. 2023. PMID: 37140079 Free PMC article. Review.
-
Transforming 2D azolium salts to 3D caged tertiary amines via stereoselective dearomative cascade annulation.Chem Sci. 2025 Mar 26;16(17):7551-7559. doi: 10.1039/d5sc01527h. eCollection 2025 Apr 30. Chem Sci. 2025. PMID: 40171030 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

