Newly synthesised oxime and lactone derivatives from Dipterocarpus alatus dipterocarpol as anti-diabetic inhibitors: experimental bioassay-based evidence and theoretical computation-based prediction
- PMID: 35492788
- PMCID: PMC9043233
- DOI: 10.1039/d1ra04461c
Newly synthesised oxime and lactone derivatives from Dipterocarpus alatus dipterocarpol as anti-diabetic inhibitors: experimental bioassay-based evidence and theoretical computation-based prediction
Abstract
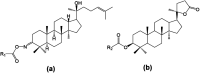
Dipterocarpus alatus-derived products are expected to exhibit anti-diabetes properties. Natural dipterocarpol (1) was isolated from Dipterocarpus alatus collected in Quang Nam province, Vietnam; afterwards, 20 derivatives including 13 oxime esters (2 and 3a-3m) and 7 lactones (4, 5, 6a-6e) were semi-synthesised. Their inhibitory effects towards diabetes-related proteins were investigated experimentally (α-glucosidase) and computationally (3W37, 3AJ7, and PTP1B). Except for compound 2, the other 19 compounds (3a-3m, 4, 5, and 6a-6d) are reported for the first time, which were modified at positions C-3, C-24 and C-25 of the dipterocarpol via imidation, esterification, oxidative cleavage and lactonisation reactions. A framework based on docking-QSARIS combination was proposed to predict the inhibitory behaviour of the ligand-protein complexes. Enzyme assays revealed the most effective α-glucosidase inhibitors, which follow the order 5 (IC50 of 2.73 ± 0.05 μM) > 6c (IC50 of 4.62 ± 0.12 μM) > 6e (IC50 of 7.31 ± 0.11 μM), and the computation-based analysis confirmed this, i.e., 5 (mass: 416.2 amu; polarisability: 52.4 Å3; DS: -14.9 kcal mol-1) > 6c (mass: 490.1 amu; polarisability: 48.8 Å3; DS: -13.7 kcal mol-1) > 6e (mass: 549.2 amu; polarisability: 51.6 Å3; DS: -15.2 kcal mol-1). Further theoretical justifications predicted 5 and 6c as versatile anti-diabetic inhibitors. The experimental results encourage next stages for the development of anti-diabetic drugs and the computational strategy invites more relevant work for validation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






Similar articles
-
Isolation, semi-synthesis, docking-based prediction, and bioassay-based activity of Dolichandrone spathacea iridoids: new catalpol derivatives as glucosidase inhibitors.RSC Adv. 2021 Mar 23;11(20):11959-11975. doi: 10.1039/d1ra00441g. eCollection 2021 Mar 23. RSC Adv. 2021. PMID: 35423771 Free PMC article.
-
Dipterocarpol in Oleoresin of Dipterocarpus alatus Attributed to Cytotoxicity and Apoptosis-Inducing Effect.Molecules. 2022 May 17;27(10):3187. doi: 10.3390/molecules27103187. Molecules. 2022. PMID: 35630669 Free PMC article.
-
Stacked ensemble learning on HaCaT cytotoxicity for skin irritation prediction: A case study on dipterocarpol.Food Chem Toxicol. 2023 Nov;181:114115. doi: 10.1016/j.fct.2023.114115. Epub 2023 Oct 18. Food Chem Toxicol. 2023. PMID: 37863382
-
Oligostilbenoids with acetylcholinesterase inhibitory activity from Dipterocarpus alatus.Planta Med. 2014 Nov;80(17):1641-6. doi: 10.1055/s-0034-1383194. Epub 2014 Oct 15. Planta Med. 2014. PMID: 25317771
-
Pyrano[3,2-c]quinoline Derivatives as New Class of α-glucosidase Inhibitors to Treat Type 2 Diabetes: Synthesis, in vitro Biological Evaluation and Kinetic Study.Med Chem. 2019;15(1):8-16. doi: 10.2174/1573406414666180528110104. Med Chem. 2019. PMID: 29807519 Review.
Cited by
-
Novel Anti-Acetylcholinesterase Effect of Euonymus laxiflorus Champ. Extracts via Experimental and In Silico Studies.Life (Basel). 2023 May 30;13(6):1281. doi: 10.3390/life13061281. Life (Basel). 2023. PMID: 37374064 Free PMC article.
References
-
- World Health Organization, Global report on diabetes, WHO Press, Geneva, Switzeland, 2016
-
- Ha M. T. Seong S. H. Nguyen T. D. Cho W. K. Ah K. J. Ma J. Y. Woo M. H. Choi J. S. Min B. S. Chalcone derivatives from the root bark of Morus alba L. act as inhibitors of PTP1B and α-glucosidase. Phytochemistry. 2018;155:114–125. doi: 10.1016/j.phytochem.2018.08.001. doi: 10.1016/j.phytochem.2018.08.001. - DOI - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

