Structural characterization, anti-inflammatory and glycosidase inhibitory activities of two new polysaccharides from the root of Pueraria lobata
- PMID: 35492792
- PMCID: PMC9043251
- DOI: 10.1039/d1ra07385k
Structural characterization, anti-inflammatory and glycosidase inhibitory activities of two new polysaccharides from the root of Pueraria lobata
Abstract
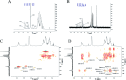
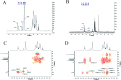
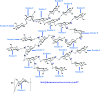
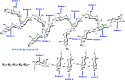
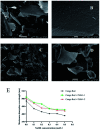
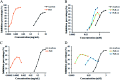
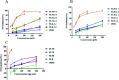
Diabetes seriously endangers public health and brings a heavy economic burden to the country. Inflammation is one of the main inducing factors of type-2 diabetes (T2D) and may cause some complications of diabetes, such as diabetic encephalopathy and peripheral neuropathy. In-depth research and development of drugs to cure diabetes and complications are of great significance. Pueraria lobate is a medicinal herb used in several countries to treat many diseases. Here, two new polysaccharides (PLB-1-1 and PLB-1-2) were isolated and purified from the root of Pueraria lobata with molecular weights of 9.1 × 103 Da and 3.8 × 103 Da, respectively. The structure was evaluated by monosaccharide composition, GC-MS and NMR spectroscopy. It was determined that PLB-1-1 comprised →4)-α-d-Glcp-(1→, α-d-Glcp-(1→, →6)-β-d-Galp-(1→, →3)-α-l-Araf-(1→, →3,6)-β-d-Manp-(1→ and →4,6)-β-d-Manp-(1→, and PLB-1-2 consisted of →4)-α-d-Glcp-(1→, β-d-Glcp-(1→, →4,6)-β-d-Glcp-(1→, →3,6)-β-d-Manp-(1→ and α-l-Fucp-(1→. Furthermore, both PLB-1-1 and PLB-1-2 showed anti-inflammatory and inhibitory activities of α-glucosidase and α-amylase in vitro. Therefore, the new polysaccharides, i.e., PLB-1-1 and PLB-1-2, may be considered candidates for the treatment of diabetes and its related complications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

