Copper-mediated construction of benzothieno[3,2- b]benzofurans by intramolecular dehydrogenative C-O coupling reaction
- PMID: 35492801
- PMCID: PMC9043471
- DOI: 10.1039/d1ra06985c
Copper-mediated construction of benzothieno[3,2- b]benzofurans by intramolecular dehydrogenative C-O coupling reaction
Abstract
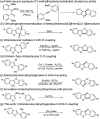
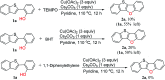
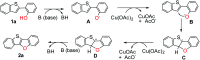
An efficient method to synthesize benzothieno[3,2-b]benzofurans via intramolecular dehydrogenative C-H/O-H coupling has been developed. Good to excellent yields (64-91%) could be obtained no matter if the substituted group is electron-donating or electron-withdrawing. Notably, three-to-six fused ring thienofuran compounds could be constructed using this method. A reaction mechanism study showed that 1,1-diphenylethylene can completely inhibit the reaction. Therefore, it is a radical pathway initiated by single electron transfer between the hydroxyl of the substrate and the copper catalyst.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Cobalt(III)-Catalyzed Intramolecular Cross-Dehydrogenative C-H/X-H Coupling: Efficient Synthesis of Indoles and Benzofurans.Chemistry. 2016 Nov 2;22(45):16042-16046. doi: 10.1002/chem.201604111. Epub 2016 Sep 30. Chemistry. 2016. PMID: 27608137
-
Copper-catalyzed intramolecular cross dehydrogenative coupling approach to coumestans from 2'-hydroxyl-3-arylcoumarins.RSC Adv. 2019 Jun 3;9(30):17391-17398. doi: 10.1039/c9ra01909j. eCollection 2019 May 29. RSC Adv. 2019. PMID: 35519854 Free PMC article.
-
Copper-Catalyzed Intramolecular Olefinic C(sp2)-H Amidation for the Synthesis of γ-Alkylidene-γ-lactams.Molecules. 2023 Sep 18;28(18):6682. doi: 10.3390/molecules28186682. Molecules. 2023. PMID: 37764458 Free PMC article.
-
Fused Heteroaromatic Rings via Metal-Mediated/Catalyzed Intramolecular C-H Activation: A Comprehensive Review.Top Curr Chem (Cham). 2019 Jul 23;377(4):21. doi: 10.1007/s41061-019-0246-3. Top Curr Chem (Cham). 2019. PMID: 31332546 Review.
-
Recent advances in copper-catalyzed dehydrogenative functionalization via a single electron transfer (SET) process.Chem Soc Rev. 2012 May 7;41(9):3464-84. doi: 10.1039/c2cs15323h. Epub 2012 Feb 20. Chem Soc Rev. 2012. PMID: 22349590 Review.
Cited by
-
Frontiers in bond construction: innovative approaches to C-C and carbon-heteroatom (C-S, C-N, C-O, C-Se, C-P) transformations-a review.RSC Adv. 2025 Jul 10;15(29):23704-23759. doi: 10.1039/d5ra03124a. eCollection 2025 Jul 4. RSC Adv. 2025. PMID: 40642459 Free PMC article. Review.
-
A Comprehensive Review on Benzofuran Synthesis Featuring Innovative and Catalytic Strategies.ACS Omega. 2024 May 6;9(19):20728-20752. doi: 10.1021/acsomega.4c02677. eCollection 2024 May 14. ACS Omega. 2024. PMID: 38764672 Free PMC article. Review.
-
Compositing Benzothieno[3,2-b]Benzofuran Derivatives with Single-Walled Carbon Nanotubes for Enhanced Thermoelectric Performance.Molecules. 2023 Sep 8;28(18):6519. doi: 10.3390/molecules28186519. Molecules. 2023. PMID: 37764295 Free PMC article.
References
LinkOut - more resources
Full Text Sources

