AXL targeting restores PD-1 blockade sensitivity of STK11/LKB1 mutant NSCLC through expansion of TCF1+ CD8 T cells
- PMID: 35492873
- PMCID: PMC9040166
- DOI: 10.1016/j.xcrm.2022.100554
AXL targeting restores PD-1 blockade sensitivity of STK11/LKB1 mutant NSCLC through expansion of TCF1+ CD8 T cells
Abstract
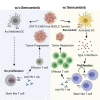
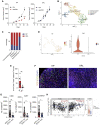
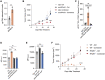
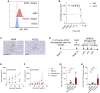
Mutations in STK11/LKB1 in non-small cell lung cancer (NSCLC) are associated with poor patient responses to immune checkpoint blockade (ICB), and introduction of a Stk11/Lkb1 (L) mutation into murine lung adenocarcinomas driven by mutant Kras and Trp53 loss (KP) resulted in an ICB refractory syngeneic KPL tumor. Mechanistically this occurred because KPL mutant NSCLCs lacked TCF1-expressing CD8 T cells, a phenotype recapitulated in human STK11/LKB1 mutant NSCLCs. Systemic inhibition of Axl results in increased type I interferon secretion from dendritic cells that expanded tumor-associated TCF1+PD-1+CD8 T cells, restoring therapeutic response to PD-1 ICB in KPL tumors. This was observed in syngeneic immunocompetent mouse models and in humanized mice bearing STK11/LKB1 mutant NSCLC human tumor xenografts. NSCLC-affected individuals with identified STK11/LKB1 mutations receiving bemcentinib and pembrolizumab demonstrated objective clinical response to combination therapy. We conclude that AXL is a critical targetable driver of immune suppression in STK11/LKB1 mutant NSCLC.
Trial registration: ClinicalTrials.gov NCT03184571.
Keywords: Axl; NSCLC; STK11/LKB1 mutation; TCF1 CD8 T cells; immunotherapy.
© 2022 The Author(s).
Conflict of interest statement
This work was supported in part by a sponsored research agreement from BerGenBio ASA to R.A.B. A.R., D.M., H.G., and G.G. are current employees of BerGenBio ASA and J.B.L. is a former employee of BerGenBio ASA. M.C. is a current employee of Merck &Co., Inc., Kenilworth, NJ. J.D.M. receives licensing royalties from the NIH and UTSW for distribution of human tumor lines. H.L., Z.L., D.M., J.B.L., J.D.M., and R.A.B. are authors of a patent related to this study. The remaining authors have no competing interests.
Figures


References
-
- Gandhi L., Rodriguez-Abreu D., Gadgeel S., Esteban E., Felip E., De Angelis F., Domine M., Clingan P., Hochmair M.J., Powell S.F., et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 2018;378:2078–2092. - PubMed
-
- Garon E.B., Rizvi N.A., Hui R., Leighl N., Balmanoukian A.S., Eder J.P., Patnaik A., Aggarwal C., Gubens M., Horn L., et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015;372:2018–2028. - PubMed
-
- Jeanson A., Tomasini P., Souquet-Bressand M., Brandone N., Boucekine M., Grangeon M., Chaleat S., Khobta N., Milia J., Mhanna L., et al. Efficacy of immune checkpoint inhibitors in KRAS-mutant non-small cell lung cancer (NSCLC) J. Thorac. Oncol. 2019;14:1095–1101. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

