T cell-attracting CCL18 chemokine is a dominant rejection signal during limb transplantation
- PMID: 35492875
- PMCID: PMC9040185
- DOI: 10.1016/j.xcrm.2022.100559
T cell-attracting CCL18 chemokine is a dominant rejection signal during limb transplantation
Abstract
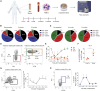
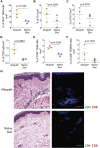
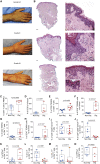
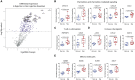
Limb transplantation is a life-changing procedure for amputees. However, limb recipients have a 6-fold greater rejection rate than solid organ transplant recipients, related in part to greater immunogenicity of the skin. Here, we report a detailed immunological and molecular characterization of individuals who underwent bilateral limb transplantation at our institution. Circulating Th17 cells are increased in limb transplant recipients over time. Molecular characterization of 770 genes in skin biopsies reveals upregulation of T cell effector immune molecules and chemokines, particularly CCL18. Skin antigen-presenting cells primarily express the chemokine CCL18, which binds to the CCR8 receptor. CCL18 treatment recruits more allo-T cells to the skin xenograft in a humanized skin transplantation model, leading to signs of accelerated graft rejection. Blockade of CCR8 remarkedly decreases CCL18-induced allo-T cell infiltration. Our results suggest that targeting the CCL18:CCR8 pathway could be a promising immunosuppressive approach in transplantation.
Trial registration: ClinicalTrials.gov NCT01293214.
Keywords: CCL18; CCR8; chemokines; extremity; limb transplantation; rejection; upper extremity transplantation; vascular composite allograft.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Murray J.E. Organ transplantation (skin, kidney, heart) and the plastic surgeon. Plast. Reconstr. Surg. 1971;47:425–431. - PubMed
-
- Kollar B., Pomahac B., Riella L.V. Novel immunological and clinical insights in vascularized composite allotransplantation. Curr. Opin. Organ Transpl. 2019;24:42–48. - PubMed
-
- Hein R.E., Ruch D.S., Klifto C.S., Leversedge F.J., Mithani S.K., Pidgeon T.S., Richard M.J., Cendales L.C. Hand transplantation in the United States: a review of the organ procurement and transplantation network/united network for organ sharing database. Am. J. Transpl. 2019;20:1417–1423. - PubMed
-
- Günther C., Bello-Fernandez C., Kopp T., Kund J., Carballido-Perrig N., Hinteregger S., Fassl S., Schwärzler C., Lametschwandtner G., Stingl G., et al. CCL18 is expressed in atopic dermatitis and mediates skin homing of human memory T cells. J. Immunol. 2005;174:1723–1728. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

