pH-Dependent transfer hydrogenation or dihydrogen release catalyzed by a [(η6-arene)RuCl(κ2- N, N-dmobpy)]+ complex: a DFT mechanistic understanding
- PMID: 35492899
- PMCID: PMC9050405
- DOI: 10.1039/c9ra10651k
pH-Dependent transfer hydrogenation or dihydrogen release catalyzed by a [(η6-arene)RuCl(κ2- N, N-dmobpy)]+ complex: a DFT mechanistic understanding
Abstract
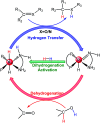
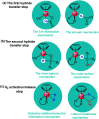
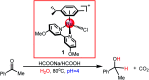
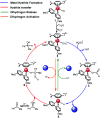
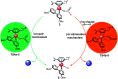
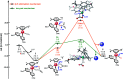
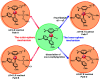
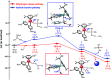
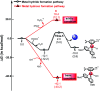
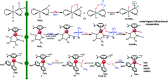
The reaction mechanism of the pH-dependent transfer hydrogenation of a ketone or the dehydrogenation of formic acid catalyzed by a [(η6-arene)RuCl(κ2-N,N-dmobpy)]+ complex in aqueous media has been investigated using the density functional theory (DFT) method. The TM-catalyzed TH of ketones with formic acid as the hydrogen source proceeds via two steps: the formation of a metal hydride and the transfer of the hydride to the substrate ketone. The calculated results show that ruthenium hydride formation is the rate-determining step. This proceeds via an ion-pair mechanism with an energy barrier of 14.1 kcal mol-1. Interestingly, the dihydrogen release process of formic acid and the hydride transfer process that produces alcohols are competitive under different pH environments. The investigation explores the feasibility of the two pathways under different pH environments. Under acidic conditions (pH = 4), the free energy barrier of the dihydrogen release pathway is 4.5 kcal mol-1 that is higher than that of the hydride transfer pathway, suggesting that the hydride transfer pathway is more favorable than the dihydrogen release pathway. However, under strongly acidic conditions, the dihydrogen release pathway is more favorable compared to the hydride transfer pathway. In addition, the ruthenium hydride formation pathway is less favorable than the ruthenium hydroxo complex formation pathway under basic conditions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Clapham S. E. Hadzovic A. Morris R. H. Coord. Chem. Rev. 2004;248:2201–2237. doi: 10.1016/j.ccr.2004.04.007. - DOI
-
- Liu Y. Yue X. Luo C. Zhang L. Lei M. Energy Environ. Mater. 2019:1–21.
-
- Lei M. Zhang W. Chen Y. Tang Y. Organometallics. 2010;29:543–548. doi: 10.1021/om900434n. - DOI
-
- Zassinovich G. Mestroni G. Gladiali S. Chem. Rev. 1992;92:1051–1069. doi: 10.1021/cr00013a015. - DOI
-
- de Graauw C. F. Peters J. A. van Bekkum H. Huskens J. Synthesis. 1994;1994:1007–1017. doi: 10.1055/s-1994-25625. - DOI
LinkOut - more resources
Full Text Sources

