Synthesis and application of coumarin fluorescence probes
- PMID: 35492912
- PMCID: PMC9050418
- DOI: 10.1039/c9ra10290f
Synthesis and application of coumarin fluorescence probes
Abstract
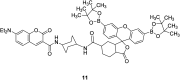
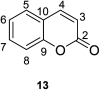
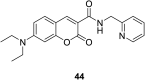
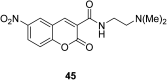
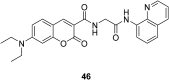
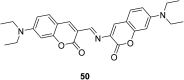
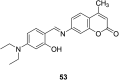
In recent years, the research on fluorescent probes has developed rapidly. Coumarin fluorescent probes have also been one of the hot topics in recent years. For the synthesis and application of coumarin fluorescent probes, great progress has been made. Coumarin fluorescent probes have become more and more widely used in biochemistry, environmental protection, and disease prevention, and have broad prospects. This review introduces the three main light emitting mechanisms (PET, ICT, FRET) of fluorescent probes, and enumerates some probes based on this light emitting mechanism. In terms of the synthesis of coumarin fluorescent probes, the existing substituents on the core of coumarin compounds were modified. Based on the positions of the modified substituents, some of the fluorescent probes reported in the past ten years are listed. Most of the fluorescent probes are formed by modifying the 3 and 7 position substituents on the mother nucleus, and the 4 and 8 position substituents are relatively less modified. In terms of probe applications, the detection and application of coumarin fluorescent probes for Cu2+, Hg2+, Mg2+, Zn2+, pH, environmental polarity, and active oxygen and sulfide in the past ten years are mainly introduced.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that this article content has no conflict of interest.
Figures
















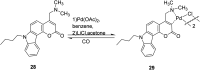

















Similar articles
-
Design, Synthesis and Application in Analytical Chemistry of Photo-Sensitive Probes Based on Coumarin.Crit Rev Anal Chem. 2021;51(6):565-581. doi: 10.1080/10408347.2020.1753163. Epub 2020 Apr 21. Crit Rev Anal Chem. 2021. PMID: 32314589 Review.
-
Design and synthesis of coumarin-based Zn(2+) probes for ratiometric fluorescence imaging.Inorg Chem. 2009 Aug 17;48(16):7630-8. doi: 10.1021/ic900247r. Inorg Chem. 2009. PMID: 19591460
-
Coumarin-Modified Starch Fluorescent Nanoparticles as Sensor of Fe3+ and Zn2+ ions Utilizing Dynamic Quenching and Chelation Mechanisms.J Fluoresc. 2025 May;35(5):3227-3238. doi: 10.1007/s10895-024-03752-3. Epub 2024 May 13. J Fluoresc. 2025. PMID: 38739316
-
Activity-Based Sensing and Theranostic Probes Based on Photoinduced Electron Transfer.Acc Chem Res. 2019 Oct 15;52(10):2818-2831. doi: 10.1021/acs.accounts.9b00340. Epub 2019 Sep 20. Acc Chem Res. 2019. PMID: 31538473 Review.
-
A New Fluorescent "Turn-Off" Coumarin-Based Chemosensor: Synthesis, Structure and Cu-Selective Fluorescent Sensing in Water Samples.J Fluoresc. 2017 Jul;27(4):1293-1298. doi: 10.1007/s10895-017-2062-x. Epub 2017 Mar 10. J Fluoresc. 2017. PMID: 28283898
Cited by
-
Visible Tracking of Small Molecules of Gases with Fluorescent Donors.Chem Biomed Imaging. 2024 Apr 1;2(6):401-412. doi: 10.1021/cbmi.4c00006. eCollection 2024 Jun 24. Chem Biomed Imaging. 2024. PMID: 39474517 Free PMC article. Review.
-
Coumarins by Direct Annulation: β-Borylacrylates as Ambiphilic C3 -Synthons.Angew Chem Int Ed Engl. 2021 Jan 11;60(2):685-689. doi: 10.1002/anie.202012099. Epub 2020 Nov 9. Angew Chem Int Ed Engl. 2021. PMID: 32975367 Free PMC article.
-
Recent Advances in Fluorescent Theranostics for Alzheimer's Disease: A Comprehensive Survey on Design, Synthesis, and Properties.ACS Omega. 2024 Mar 11;9(12):13556-13591. doi: 10.1021/acsomega.3c10417. eCollection 2024 Mar 26. ACS Omega. 2024. PMID: 38559945 Free PMC article. Review.
-
Self-Warning and Self-Repairing Mechanisms in Functional Coatings: A Review.Exploration (Beijing). 2025 Mar 7;5(4):e20240066. doi: 10.1002/EXP.20240066. eCollection 2025 Aug. Exploration (Beijing). 2025. PMID: 40873636 Free PMC article. Review.
-
Fluorescent probes for investigating the internalisation and action of bioorthogonal ruthenium catalysts within Gram-positive bacteria.RSC Chem Biol. 2024 Oct 15;5(12):1201-13. doi: 10.1039/d4cb00187g. Online ahead of print. RSC Chem Biol. 2024. PMID: 39421717 Free PMC article.
References
-
- Li X. Q. Gu J. P. Zhou Z. Ma L. F. Zheng Y. H. Tang Y. P. Gao J. W. Wang Q. M. Chem. Eng. J. 2019;358:67–73. doi: 10.1016/j.cej.2018.10.003. - DOI
-
- Deshmukh P. P. Navalkar A. Maji S. K. Manjare S. T. Sens. Actuators, B. 2018;281:8–13. doi: 10.1016/j.snb.2018.10.072. - DOI
-
- Wu N. T. Tang Y. P. Zeng M. Gao J. W. Lu X. B. Zheng Y. H. J. Lumin. 2018;202:502–507. doi: 10.1016/j.jlumin.2018.06.018. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

