Synthesis of α-indolylacrylates as potential anticancer agents using a Brønsted acid ionic liquid catalyst and the butyl acetate solvent
- PMID: 35493022
- PMCID: PMC9051412
- DOI: 10.1039/d0ra00990c
Synthesis of α-indolylacrylates as potential anticancer agents using a Brønsted acid ionic liquid catalyst and the butyl acetate solvent
Abstract
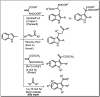
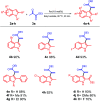
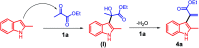
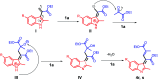
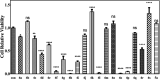
In this study, new α-indolylacrylate derivatives were synthesized by the reaction of 2-substituted indoles with various pyruvates using a Brønsted acid ionic liquid catalyst in butyl acetate solvent. This is the first report on the application of pyruvate compounds for the synthesis of indolylacrylates. The acrylate derivatives could be obtained in good to excellent yields. A preliminary biological evaluation revealed their promising anticancer activity (IC50 = 9.73 μM for the compound 4l) and indicated that both the indole core and the acrylate moieties are promising for the development of novel anticancer drugs. The Lipinski's rule and Veber's parameters were assessed for the newly synthesized derivatives.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no competing financial interest.
Figures





References
-
- Finefield J. M. Frisvad J. C. Sherman D. H. Williams R. M. J. Nat. Prod. 2012;75:812–833. doi: 10.1021/np200954v. - DOI - PMC - PubMed
- Shiri M. Chem. Rev. 2012;112:3508–3549. doi: 10.1021/cr2003954. - DOI - PubMed
- Shiri M. Zolfigol M. A. Kruger H. G. Tanbakouchian Z. Chem. Rev. 2010;110:2250–2293. doi: 10.1021/cr900195a. - DOI - PubMed
- Humphrey G. R. Kuethe J. T. Chem. Rev. 2006;106:2875. doi: 10.1021/cr0505270. - DOI - PubMed
- Bhadury P. S. Pang J. Curr. Org. Chem. 2014;18:2108–2124. doi: 10.2174/1385272819666140809010818. - DOI
-
- Fantacuzzi M. De Filippis B. Gallorini M. Ammazzalorso A. Giampietro L. Maccallini C. Aturki Z. Donati E. Ibrahim R. S. Shawky E. Cataldi A. Amoroso R. Eur. J. Med. Chem. 2020;185:111815. doi: 10.1016/j.ejmech.2019.111815. - DOI - PubMed
- Al-Wabli R. I. Almomen A. A. Almutairi M. S. Keeton A. B. Piazza G. A. Attia M. I. Drug Des. Dev. Ther. 2020;14:483–495. doi: 10.2147/DDDT.S227862. - DOI - PMC - PubMed
- Demurtas M. Baldisserotto A. Lampronti I. Moi D. Balboni G. Pacifico S. Vertuani S. Manfredini S. Onnis V. Bioorg. Chem. 2019;85:568–576. doi: 10.1016/j.bioorg.2019.02.007. - DOI - PubMed
- Cury N. M. Capitão R. M. de Almeida R. do C. B. Artico L. L. Corrêa J. R. Simão dos Santos E. F. Yunes J. A. Correia C. R. D. Eur. J. Med. Chem. 2019;181:111570. doi: 10.1016/j.ejmech.2019.111570. - DOI - PubMed
- Li W. Sun H. Xu F. Shuai W. Liu J. Xu S. Yao H. Ma C. Zhu Z. Xu J. Bioorg. Chem. 2019;85:49–59. doi: 10.1016/j.bioorg.2018.12.015. - DOI - PubMed
- La Regina G. Bai R. Coluccia A. Naccarato V. Famiglini V. Nalli M. Masci D. Verrico A. Rovella P. Mazzoccoli C. Da Pozzo E. Cavallini C. Martini C. Vultaggio S. Dondio G. Varasi M. Mercurio C. Hamel E. Lavia P. Silvestri R. Eur. J. Med. Chem. 2018;152:283–297. doi: 10.1016/j.ejmech.2018.04.042. - DOI - PubMed
-
- Khan M. F. Anwer T. Bakht A. Verma G. Akhtar W. Alam M. M. Rizvi M. A. Akhter M. Shaquiquzzaman M. Bioorg. Chem. 2019;87:667–678. doi: 10.1016/j.bioorg.2019.03.071. - DOI - PubMed
- Vlaicu I. D. Olar R. Maxim C. Chifiriuc M. C. Bleotu C. Stănică N. Vasile Scăeţeanu G. Dulea C. Avram S. Badea M. Appl. Organomet. Chem. 2019;33:1–13. doi: 10.1002/aoc.4976. - DOI
- Verma G. Chashoo G. Ali A. Khan M. F. Akhtar W. Ali I. Akhtar M. Alam M. M. Shaquiquzzaman M. Bioorg. Chem. 2018;77:106–124. doi: 10.1016/j.bioorg.2018.01.007. - DOI - PubMed
- Fang S. Chen L. Yu M. Cheng B. Lin Y. Morris-Natschke S. L. Lee K. H. Gu Q. Xu J. Org. Biomol. Chem. 2015;13:4714–4726. doi: 10.1039/C5OB00007F. - DOI - PMC - PubMed
- Yu-Jen C. Shiao M.-S. Hsu M.-L. Tsai T.-H. Sheng-Yuan W. J. Agric. Food Chem. 2001;49:5615–5619. doi: 10.1021/jf0107252. - DOI - PubMed
-
- Arcadi A. Bianchi G. Chiarini M. D'Anniballe G. Marinelli F. Synlett. 2004;6:944–950. doi: 10.1055/s-2004-822903. - DOI
LinkOut - more resources
Full Text Sources

