Photocatalytic cascade reactions and dye degradation over CdS-metal-organic framework hybrids
- PMID: 35493156
- PMCID: PMC9043023
- DOI: 10.1039/d1ra05957b
Photocatalytic cascade reactions and dye degradation over CdS-metal-organic framework hybrids
Abstract
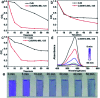
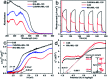
Two bifunctional CdS-MOF composites have been designed and fabricated. The hybrids exhibited synergistic photocatalytic performance toward two cascade reactions under visible light integrating photooxidation activity of CdS and Lewis acids/bases of the MOF. The composite further promoted the photodegradation of dyes benefiting from effective electron transfer between the MOF and CdS.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Mehta V. P. Eycken E. V. V. Chem. Soc. Rev. 2011;40:4925–4936. doi: 10.1039/C1CS15094D. - DOI - PubMed
- Tietze L. F. Chem. Rev. 1996;96:115–136. doi: 10.1021/cr950027e. - DOI - PubMed
- List B. Angew. Chem., Int. Ed. 2010;49:1730–1734. doi: 10.1002/anie.200906900. - DOI - PubMed
- Gnanaprakasam B. Zhang J. Milstein D. Angew. Chem., Int. Ed. 2010;49:1468–1471. doi: 10.1002/anie.200907018. - DOI - PubMed
- Shiroodi R. K. Gevorgyan V. Chem. Soc. Rev. 2013;42:4991–5001. doi: 10.1039/C3CS35514D. - DOI - PMC - PubMed
- Li X. Lin B. Li H. Yu Q. Ge Y. Jin X. Liu X. Zhou Y. Xiao J. Appl. Catal., B. 2018;239:254–259. doi: 10.1016/j.apcatb.2018.08.021. - DOI
- Wu R. Wang S. Zhou Y. Long J. Dong F. Zhang W. ACS Appl. Nano Mater. 2019;2:6818–6827. doi: 10.1021/acsanm.9b01264. - DOI
-
- Zhu J.-J. Kailasam K. Fischer A. Thomas A. ACS Catal. 2011;1:342–347. doi: 10.1021/cs100153a. - DOI
- Wu P.-P. Cao Y. X. Zhao L. M. Wang Y. He Z. K. Xing W. Bai P. Mintova S. Yan Z. F. J. Catal. 2019;375:32–43. doi: 10.1016/j.jcat.2019.05.003. - DOI
- Tran U. P. N. Le K. K. A. Phan N. T. S. ACS Catal. 2011;1:120–127. doi: 10.1021/cs1000625. - DOI
- Rashidizadeh A. Zand H. R. E. Ghafuri H. Rezazadeh Z. ACS Appl. Nano Mater. 2020;3:7057–7065. doi: 10.1021/acsanm.0c01380. - DOI
-
- Xu Q. Cheng B. Yu J. Liu G. Carbon. 2017;118:241–249. doi: 10.1016/j.carbon.2017.03.052. - DOI
- Wu Y.-P. Yang B. Tian J. Yu S.-B. Wang H. Zhang D.-W. Liu Y. Li Z.-T. Chem. Commun. 2017;53:13367–13370. doi: 10.1039/C7CC08824H. - DOI - PubMed
- Li Y. Liu M. Chen L. J. Mater. Chem. A. 2017;5:13757–13762. doi: 10.1039/C7TA03776G. - DOI
- Meng S. Ning X. Chang S. Fu X. Ye X. Chen S. J. Catal. 2018;357:247–256. doi: 10.1016/j.jcat.2017.11.015. - DOI
- Zhang Y. Zhou J. Chen J. Feng X. Cai W. J. Hazard. Mater. 2020;392:122315. doi: 10.1016/j.jhazmat.2020.122315. - DOI - PubMed
-
- Cheng L. Xiang Q. Liao Y. Zhang H. Energy Environ. Sci. 2018;11:1362–1391. doi: 10.1039/C7EE03640J. - DOI
LinkOut - more resources
Full Text Sources

