Graphene oxide-catalyzed trifluoromethylation of alkynes with quinoxalinones and Langlois' reagent
- PMID: 35493205
- PMCID: PMC9044184
- DOI: 10.1039/d1ra07014b
Graphene oxide-catalyzed trifluoromethylation of alkynes with quinoxalinones and Langlois' reagent
Abstract
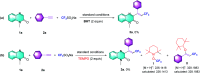
The direct C-H trifluoromethylation of alkynes and quinoxalinones has been achieved using a graphene oxide/Langlois' reagent system. This multi-component tandem reaction using graphene oxide as the catalyst and Langlois' reagent as the robust CF3 radical source results in the formation of olefinic C-CF3 to access a series of 3-trifluoroalkylated quinoxalin-2(1H)-ones.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Wu H. Su C. Tandiana R. Liu C. Qiu C. Bao Y. Wu J. Xu Y. Lu J. Fan D. Loh K. P. Angew. Chem., Int. Ed. 2018;57:10848. doi: 10.1002/anie.201802548. - DOI - PubMed
- Tan C. Cao X. Wu X.-J. He Q. Yang J. Zhang X. Chen J. Zhao W. Han S. Nam G.-H. Sindoro M. Zhang H. Chem. Rev. 2017;117:6225. doi: 10.1021/acs.chemrev.6b00558. - DOI - PubMed
- Chua C. K. Pumera M. Chem.–Eur. J. 2015;21:12550. doi: 10.1002/chem.201501383. - DOI - PubMed
- Dreyer D. R. Todd A. D. Bielawski C. W. Chem. Soc. Rev. 2014;43:5288. doi: 10.1039/C4CS00060A. - DOI - PubMed
- Navalon S. Dhakshinamoorthy A. Alvaro M. Garcia H. Chem. Rev. 2014;114:6179. doi: 10.1021/cr4007347. - DOI - PubMed
- Su C. Loh K. P. Acc. Chem. Res. 2013;46:2275. doi: 10.1021/ar300118v. - DOI - PubMed
-
- Soni J. Sethiya A. Sahiba N. Agarwal S. Appl. Organomet. Chem. 2021:e6162.
- Lombardi L. Bandini M. Angew. Chem., Int. Ed. 2020;59:20767. doi: 10.1002/anie.202006932. - DOI - PubMed
- Bahuguna A. Kumar A. Krishnan V. Asian J. Org. Chem. 2019;8:1263. doi: 10.1002/ajoc.201900259. - DOI
- Zhang J. Chen S. Chen F. Xu W. Gong H. Adv. Synth. Catal. 2017;359:2358. doi: 10.1002/adsc.201700178. - DOI
- Tang P. Hu G. Li M. Ma D. ACS Catal. 2016;6:6948. doi: 10.1021/acscatal.6b01668. - DOI
- Koehler F. M. Stark W. J. Acc. Chem. Res. 2013;46:2297. doi: 10.1021/ar300125w. - DOI - PubMed
- Su C. Acik M. Takai K. Lu J. Hao S. Zheng Y. Wu P. Bao Q. Enoki T. Chabal Y. J. Loh K. P. Nat. Commun. 2012:1298. doi: 10.1038/ncomms2315. - DOI - PubMed
- Dhakshinamoorthy A. Alvaro M. Concepción P. Fornés V. Garcia H. Chem. Commun. 2012;48:5443. doi: 10.1039/C2CC31385E. - DOI - PubMed
- Su D. S. Zhang J. Frank B. Thomas A. Wang X. Paraknowitsch J. Schlögl R. ChemSusChem. 2010;3:169. doi: 10.1002/cssc.200900180. - DOI - PubMed
-
- Yeh T.-F. Teng C.-Y. Chen L.-C. Chen S.-J. Teng H. J. Mater. Chem. A. 2016;4:2014. doi: 10.1039/C5TA07780J. - DOI
- Zhang N. Yang M.-Q. Liu S. Sun Y. Xu Y.-J. Chem. Rev. 2015;115:10307. doi: 10.1021/acs.chemrev.5b00267. - DOI - PubMed
- Roy-Mayhew J. D. Aksay I. A. Chem. Rev. 2014;114:6323. doi: 10.1021/cr400412a. - DOI - PubMed
- Chung C. Kim Y.-K. Shin D. Ryoo S.-Y. Hong B.-H. Min D.-H. Acc. Chem. Res. 2013;46:2211. doi: 10.1021/ar300159f. - DOI - PubMed
-
- Hu J.-P. Tian C.-Q. Damaneh M. S. Li Y.-L. Cao D.-Y. Lv K.-K. Yu T. Meng T. Chen D.-Q. Wang X. Chen L. Li J. Song S.-S. Huan X.-J. Qin L.-H. Shen J.-K. Wang Y.-Q. Miao Z.-H. Xiong B. J. Med. Chem. 2019;62:8642. doi: 10.1021/acs.jmedchem.9b01094. - DOI - PubMed
- Hussain S. Parveen S. Hao X. Zhang S. Wang W. Qin X. Yang Y. Chen X. Zhu S. Zhu C. Ma B. Eur. J. Med. Chem. 2014;80:383. doi: 10.1016/j.ejmech.2014.04.047. - DOI - PubMed
- Liu R. Huang Z.-H. Murray M. G. Guo X.-Y. Liu G. J. Med. Chem. 2011;54:5747. doi: 10.1021/jm200394x. - DOI - PubMed
-
- Schiesser S. Chepliaka H. Kollback J. Quennesson T. Czechtizky W. Cox R. J. J. Med. Chem. 2020;63:13076. doi: 10.1021/acs.jmedchem.0c01457. - DOI - PubMed
- Oh E. H. Kim H. J. Han S. B. Synthesis. 2018;50:3346. doi: 10.1055/s-0037-1610085. - DOI
- Song H.-X. Han Q.-Y. Zhao C.-L. Zhang C.-P. Green Chem. 2018;20:1662. doi: 10.1039/C8GC00078F. - DOI
- Yang X. Wu T. Phipps R. J. Toste F. D. Chem. Rev. 2015;115:826. doi: 10.1021/cr500277b. - DOI - PMC - PubMed
- Zhu W. Wang J. Wang S. Gu Z. Aceña J. L. Izawa K. Liu H. Soloshonok V. A. J. Fluorine Chem. 2014;167:37. doi: 10.1016/j.jfluchem.2014.06.026. - DOI
- Furuya T. Kamlet A. S. Ritter T. Nature. 2011;473:470. doi: 10.1038/nature10108. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources