Anthraquinone-2,6-disulfamidic acid: an anolyte with low decomposition rates at elevated temperatures
- PMID: 35493233
- PMCID: PMC9044267
- DOI: 10.1039/d1ra05545c
Anthraquinone-2,6-disulfamidic acid: an anolyte with low decomposition rates at elevated temperatures
Abstract
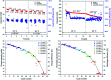
A new sulfamidic acid anthraquinone derivative was synthesized from 2,6-diaminoanthraquinone with high yields, designed for utilization in redox flow batteries. The active material was investigated with cyclic voltammetry, revealing a reversible redox reaction at approximately -0.65 V vs. Ag/AgCl at pH-values above 12. A stress test in a redox flow battery was applied with hold times at critical states of charge and at 32 °C as well as at 60 °C. Furthermore, the stability was investigated at the maximum concentration of the anolyte. All in all, the material showed the lowest decomposition rates at 60 °C reported so far for an organic anolyte in a redox flow battery.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

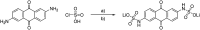


References
LinkOut - more resources
Full Text Sources

