Differences in Humoral and Cellular Vaccine Responses to SARS-CoV-2 in Kidney and Liver Transplant Recipients
- PMID: 35493446
- PMCID: PMC9047689
- DOI: 10.3389/fimmu.2022.853682
Differences in Humoral and Cellular Vaccine Responses to SARS-CoV-2 in Kidney and Liver Transplant Recipients
Abstract
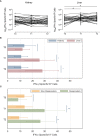
The antibody and T cell responses after SARS-CoV-2 vaccination have not been formally compared between kidney and liver transplant recipients. Using a multiplex assay, we measured IgG levels against 4 epitopes of SARS-CoV-2 spike protein and nucleocapsid (NC) antigen, SARS-CoV-2 variants, and common coronaviruses in serial blood samples from 52 kidney and 50 liver transplant recipients undergoing mRNA SARS-CoV-2 vaccination. We quantified IFN-γ/IL-2 T cells reactive against SARS-CoV-2 spike protein by FluoroSpot. We used multivariable generalized linear models to adjust for the differences in immunosuppression between groups. In liver transplant recipients, IgG levels against every SARS-CoV-2 spike epitope increased significantly more than in kidney transplant recipients (MFI: 19,617 vs 6,056; P<0.001), a difference that remained significant after adjustments. Vaccine did not affect IgG levels against NC nor common coronaviruses. Elicited antibodies recognized all variants tested but at significantly lower strength than the original Wuhan strain. Anti-spike IFN-γ-producing T cells increased significantly more in liver than in kidney transplant recipients (IFN-γ-producing T cells 28 vs 11 spots/5x105 cells), but this difference lost statistical significance after adjustments. SARS-CoV-2 vaccine elicits a stronger antibody response in liver than in kidney transplant recipients, a phenomenon that is not entirely explained by the different immunosuppression.
Keywords: COVID-19; T cell; antibody; immunosuppression; variant.
Copyright © 2022 Furian, Russo, Zaza, Burra, Hartzell, Bizzaro, Di Bello, Di Bella, Nuzzolese, Agnolon, Florman, Rana, Lee, Kim, Maggiore, Maltzman and Cravedi.
Conflict of interest statement
JM has received research support and honoraria from Thermo Fisher Scientific/One Lambda, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

