Non-Muscle Myosin II Is Essential for the Negative Regulation of B-Cell Receptor Signaling and B-Cell Activation
- PMID: 35493485
- PMCID: PMC9047714
- DOI: 10.3389/fimmu.2022.842605
Non-Muscle Myosin II Is Essential for the Negative Regulation of B-Cell Receptor Signaling and B-Cell Activation
Abstract
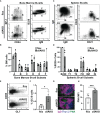
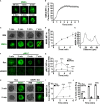
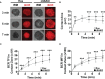
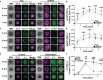
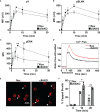
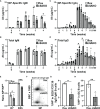
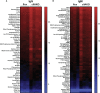
Antigen (Ag)-triggered B-cell receptor (BCR) signaling initiates antibody responses. However, prolonged or uncontrolled BCR signaling is associated with the development of self-reactive B-cells and autoimmune diseases. We previously showed that actin-mediated B-cell contraction on Ag-presenting surfaces negatively regulates BCR signaling. Non-muscle myosin II (NMII), an actin motor, is involved in B-cell development and antibody responses by mediating B-cell migration, cytokinesis, and Ag extraction from Ag-presenting cells. However, whether and how NMII regulates humoral responses through BCR signaling remains elusive. Utilizing a B-cell-specific, partial NMIIA knockout (cIIAKO) mouse model and NMII inhibitors, this study examined the role of NMII in BCR signaling. Upon BCR binding to antibody-coated planar lipid bilayers (PLB), NMIIA was recruited to the B-cell contact membrane and formed a ring-like structure during B-cell contraction. NMII recruitment depended on phosphatidylinositol 5-phosphatase (SHIP1), an inhibitory signaling molecule. NMII inhibition by cIIAKO did not affect B-cell spreading on PLB but delayed B-cell contraction and altered BCR clustering. Surface BCR "cap" formation induced by soluble stimulation was enhanced in cIIAKO B-cells. Notably, NMII inhibition by cIIAKO and inhibitors up-regulated BCR signaling in response to both surface-associated and soluble stimulation, increasing phosphorylated tyrosine, CD79a, BLNK, and Erk and decreasing phosphorylated SHIP1. While cIIAKO did not affect B-cell development, the number of germinal center B-cells was significantly increased in unimmunized cIIAKO mice, compared to control mice. While cIIAKO mice mounted similar antibody responses when compared to control mice upon immunization, the percentages of high-affinity antibodies, Ag-specific germinal center B-cells and isotype switched B-cells were significantly lower in cIIAKO mice than in control mice. Furthermore, autoantibody levels were elevated in cIIAKO mice, compared to control mice. Collectively, our results reveal that NMII exerts a B-cell-intrinsic inhibition on BCR signaling by regulating B-cell membrane contraction and surface BCR clustering, which curtails the activation of non-specific and self-reactive B-cells.
Keywords: B lymphocytes; B-cell receptor; actin cytoskeleton; antibody response; non-muscle myosin II; signal transduction.
Copyright © 2022 Seeley-Fallen, Lazzaro, Liu, Li, Upadhyaya and Song.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
N-WASP-dependent branched actin polymerization attenuates B-cell receptor signaling by increasing the molecular density of receptor clusters.Elife. 2023 Dec 12;12:RP87833. doi: 10.7554/eLife.87833. Elife. 2023. PMID: 38085658 Free PMC article.
-
Actin-binding protein 1 links B-cell antigen receptors to negative signaling pathways.Proc Natl Acad Sci U S A. 2014 Jul 8;111(27):9881-6. doi: 10.1073/pnas.1321971111. Epub 2014 Jun 23. Proc Natl Acad Sci U S A. 2014. PMID: 24958882 Free PMC article.
-
A balance of Bruton's tyrosine kinase and SHIP activation regulates B cell receptor cluster formation by controlling actin remodeling.J Immunol. 2011 Jul 1;187(1):230-9. doi: 10.4049/jimmunol.1100157. Epub 2011 May 27. J Immunol. 2011. PMID: 21622861 Free PMC article.
-
The pivotal position of the actin cytoskeleton in the initiation and regulation of B cell receptor activation.Biochim Biophys Acta. 2014 Feb;1838(2):569-78. doi: 10.1016/j.bbamem.2013.07.016. Epub 2013 Jul 23. Biochim Biophys Acta. 2014. PMID: 23886914 Free PMC article. Review.
-
Bidirectional feedback between BCR signaling and actin cytoskeletal dynamics.FEBS J. 2022 Aug;289(15):4430-4446. doi: 10.1111/febs.16074. Epub 2021 Jun 30. FEBS J. 2022. PMID: 34124846 Free PMC article. Review.
Cited by
-
Background and roles: myosin in autoimmune diseases.Front Cell Dev Biol. 2023 Aug 23;11:1220672. doi: 10.3389/fcell.2023.1220672. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37691828 Free PMC article. Review.
-
N-WASP-dependent branched actin polymerization attenuates B-cell receptor signaling by increasing the molecular density of receptor clusters.Elife. 2023 Dec 12;12:RP87833. doi: 10.7554/eLife.87833. Elife. 2023. PMID: 38085658 Free PMC article.
-
Mechanical regulation of lymphocyte activation and function.J Cell Sci. 2024 Jul 1;137(13):jcs219030. doi: 10.1242/jcs.219030. Epub 2024 Jul 12. J Cell Sci. 2024. PMID: 38995113 Free PMC article. Review.
-
Ion channel TRPV2 is critical in enhancing B cell activation and function.J Exp Med. 2024 Mar 4;221(3):e20221042. doi: 10.1084/jem.20221042. Epub 2024 Feb 14. J Exp Med. 2024. PMID: 38353705 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

