PAMPs of Piscirickettsia salmonis Trigger the Transcription of Genes Involved in Nutritional Immunity in a Salmon Macrophage-Like Cell Line
- PMID: 35493529
- PMCID: PMC9046600
- DOI: 10.3389/fimmu.2022.849752
PAMPs of Piscirickettsia salmonis Trigger the Transcription of Genes Involved in Nutritional Immunity in a Salmon Macrophage-Like Cell Line
Abstract
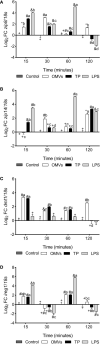
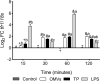
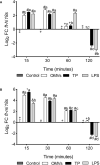
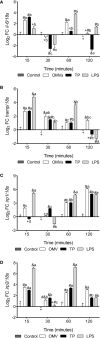
The innate immune system can limit the growth of invading pathogens by depleting micronutrients at a cellular and tissue level. However, it is not known whether nutrient depletion mechanisms discriminate between living pathogens (which require nutrients) and pathogen-associated molecular patterns (PAMPs) (which do not). We stimulated SHK-1 cells with different PAMPs (outer membrane vesicles of Piscirickettsia salmonis "OMVs", protein extract of P. salmonis "TP" and lipopolysaccharides of P. salmonis "LPS") isolated from P. salmonis and evaluated transcriptional changes in nutritional immunity associated genes. Our experimental treatments were: Control (SHK-1 stimulated with bacterial culture medium), OMVs (SHK-1 stimulated with 1μg of outer membrane vesicles), TP (SHK-1 stimulated with 1μg of total protein extract) and LPS (SHK-1 stimulated with 1μg of lipopolysaccharides). Cells were sampled at 15-, 30-, 60- and 120-minutes post-stimulation. We detected increased transcription of zip8, zip14, irp1, irp2 and tfr1 in all three experimental conditions and increased transcription of dmt1 in cells stimulated with OMVs and TP, but not LPS. Additionally, we observed generally increased transcription of ireg-1, il-6, hamp, irp1, ft-h and ft-m in all three experimental conditions, but we also detected decreased transcription of these markers in cells stimulated with TP and LPS at specific time points. Our results demonstrate that SHK-1 cells stimulated with P. salmonis PAMPs increase transcription of markers involved in the transport, uptake, storage and regulation of micronutrients such as iron, manganese and zinc.
Keywords: PAMPs (pathogen associated molecular patterns); Piscirickettsia salmonis; Salmo salar; nutritional immunology; transcription.
Copyright © 2022 Martínez, Oliver, Santibañez, Coronado, Oyarzún-Salazar, Enriquez, Vargas-Chacoff and Romero.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Barandica L, Tort L. Neuroendocrinología E Inmunología De La Respuesta Al Estrés En Peces. Rev la Acad Colomb Cienc Exactas Físicas y Nat (2008) 32:267–84.
-
- Soulliere C, Dixon B. Immune System Organs of Bony Fishes. Canada: Elsevier Ltd; (2017).
-
- Sompayrac L. How System Immune Works. Fourth. USA: John Wiley & Sons; (2012).
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

