Biomedical nanobubbles and opportunities for microfluidics
- PMID: 35493576
- PMCID: PMC9042222
- DOI: 10.1039/d1ra04890b
Biomedical nanobubbles and opportunities for microfluidics
Abstract
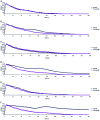
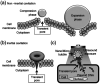
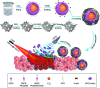
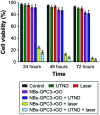
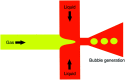
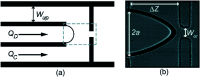
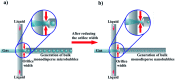
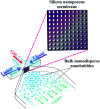
The use of bulk nanobubbles in biomedicine is increasing in recent years, which is attributable to the array of therapeutic and diagnostic tools promised by developing bulk nanobubble technologies. From cancer drug delivery and ultrasound contrast enhancement to malaria detection and the diagnosis of acute donor tissue rejection, the potential applications of bulk nanobubbles are broad and diverse. Developing these technologies to the point of clinical use may significantly impact the quality of patient care. This review compiles and summarizes a representative collection of the current applications, fabrication techniques, and characterization methods of bulk nanobubbles in biomedicine. Current state-of-the-art generation methods are not designed to create nanobubbles of high concentration and low polydispersity, both characteristics of which are important for several bulk nanobubble applications. To date, microfluidics has not been widely considered as a tool for generating nanobubbles, even though the small-scale precision and real-time control offered by microfluidics may overcome the challenges mentioned above. We suggest possible uses of microfluidics for improving the quality of bulk nanobubble populations and propose ways of leveraging existing microfluidic technologies, such as organ-on-a-chip platforms, to expand the experimental toolbox of researchers working to develop biomedical nanobubbles.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





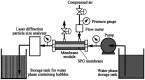
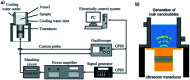



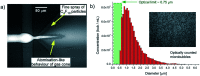
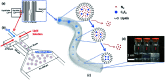
References
-
- Attard P. Moody M. P. Tyrrell J. W. G. Nanobubbles: The big picture. Phys. Stat. Mech. Appl. 2002;314(1–4):696–705. doi: 10.1016/S0378-4371(02)01191-3. - DOI
-
- Meegoda J. N. Aluthgun Hewage S. Batagoda J. H. Stability of nanobubbles. Environ. Eng. Sci. 2018;35(11):1216–1227. doi: 10.1089/ees.2018.0203. - DOI
Publication types
LinkOut - more resources
Full Text Sources

