Photo-responsive azobenzene interactions promote hierarchical self-assembly of collagen triple-helical peptides to various higher-order structures
- PMID: 35493640
- PMCID: PMC9052399
- DOI: 10.1039/d0ra02906h
Photo-responsive azobenzene interactions promote hierarchical self-assembly of collagen triple-helical peptides to various higher-order structures
Abstract
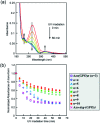
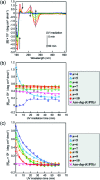
Collagen is an essential structural protein in animal tissues and plays key roles in cellular modulation. We investigated methods to discover collagen model peptides (CMPs) that would self-assemble into triple helices and then grow into supramolecular organizations with diverse morphological features, which would be valuable as biomaterials. This challenging undertaking was achieved by placing azobenzene groups on the ends of the CMPs, (GPO) n (n = 3-10), Azo-(GPO) n . In a dilute aqueous solution (80 μM), CD spectra indicated that the Azo-(GPO) n (n > 4) formed triple helices due to strong hydrophobic azobenzene interactions, and that helix stability was increased with the peptide segment length. The resulting triple helices induced a specific azobenzene orientation through turned and twisted configurations as shown by CD spectra. TEM observations for the same solutions disclosed the morphologies for the Azo-CMPs. Azo-(GPO)3, having the shortest peptide segment, showed no nanostructure, both Azo-(GPO)4 and Azo-(GPO)5 provided consistent well-developed nanofiber structures resembling the natural collagen fibers, and Azo-(GPO) n s (n = 6-10) grew into flexible rod-like micelle fibers. In addition, alkyl chain-attached C m Azo-(GPO)5 displayed a toroidal morphology, and Azp-deg-(GPO)5 having a hydrophilic spacer assembled into a bilayer vesicle structure. These diverse morphological features are considered to be due to the characteristics of the pre-organized triple helix units. Photo-isomerization of the azobenzene moiety brought about the disappearance of such characteristic nano-architectures. When the solution concentration was increased up to 1 wt%, only Azo-(GPO)4 and Azo-(GPO)5 spontaneously formed hydrogels exhibiting a satisfactory gel-to-sol transition upon UV irradiation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

