Synthesis and in vitro anti-proliferative evaluation of naphthalimide-chalcone/pyrazoline conjugates as potential SERMs with computational validation
- PMID: 35493668
- PMCID: PMC9052575
- DOI: 10.1039/d0ra01822h
Synthesis and in vitro anti-proliferative evaluation of naphthalimide-chalcone/pyrazoline conjugates as potential SERMs with computational validation
Abstract
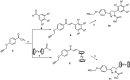
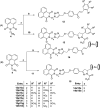
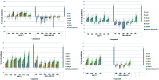
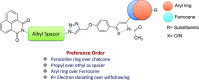
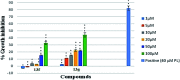
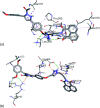
A series of naphthalimide-chalcone/pyrazoline conjugates was prepared and evaluated for their anti-breast cancer potential against estrogen responsive, i.e. MCF-7 (ER+), and triple-negative, i.e. MDA-MB-231 (ER-), cell lines. The structure-activity-relationship (SAR) was deduced based on the influence of linker length, substituents on the phenyl ring and the generated functionalities, on anti-proliferative activities. Docking simulations further delineate the type of interactions of the designed molecules with the selected targets. This report discloses the scope of triazole tethered naphthalimide-chalcone/pyrazoline conjugates as anti breast cancer agents.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
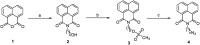
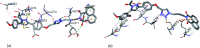
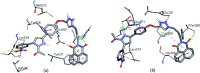
References
-
- Cancer Facts and Figures 2019, American Cancer Society, https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts...
LinkOut - more resources
Full Text Sources
Miscellaneous

