Ring-opening cyclization of activated spiro-aziridine oxindoles with heteroarenes: a facile synthetic approach to spiro-oxindole-fused pyrroloindolines
- PMID: 35493670
- PMCID: PMC9052938
- DOI: 10.1039/d0ra00684j
Ring-opening cyclization of activated spiro-aziridine oxindoles with heteroarenes: a facile synthetic approach to spiro-oxindole-fused pyrroloindolines
Abstract
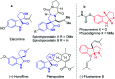
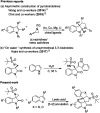
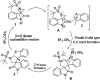
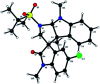
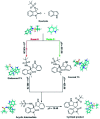
Herein, we report a facile tandem approach for the synthesis of both spiro-oxindole-fused pyrroloindolines and benzofurano-pyrrolidines via a Lewis acid-catalyzed domino ring-opening with concomitant ring annulation using activated spiro-aziridines and heteroarenes. This method offers a new class of novel spiro-fused polycyclic pyrrolidines in a one-pot and sustainable manner with good yields and high diastereoselectivity. In addition, the structure of 3d was confirmed by single X-ray crystallography analysis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
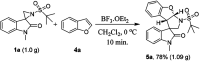
Similar articles
-
Selective C3-Allylation and Formal [3 + 2]-Annulation of Spiro-Aziridine Oxindoles: Synthesis of 5'-Substituted Spiro[pyrrolidine-3,3'-oxindoles] and Coerulescine.J Org Chem. 2022 Jul 1;87(13):8656-8671. doi: 10.1021/acs.joc.2c00863. Epub 2022 Jun 22. J Org Chem. 2022. PMID: 35731944
-
A Facile One-Pot Construction of Succinimide-Fused Spiro[Pyrrolidine-2,3'-Oxindoles] via 1,3-Dipolar Cycloaddition Involving 3-Amino Oxindoles and Maleimides.Molecules. 2018 Mar 5;23(3):582. doi: 10.3390/molecules23030582. Molecules. 2018. PMID: 29510590 Free PMC article.
-
Cascade Ring-Opening/Cyclization Reaction of Spiro(nitrocyclopropane)oxindoles with Huisgen Zwitterions and Synthesis of Pyrazolo[3,4-b]indoles.J Org Chem. 2022 Dec 16;87(24):16707-16721. doi: 10.1021/acs.joc.2c02375. Epub 2022 Dec 6. J Org Chem. 2022. PMID: 36473167
-
Syntheses and reactivity of spiro-epoxy/aziridine oxindole cores: developments in the past decade.Org Biomol Chem. 2020 Nov 4;18(42):8572-8596. doi: 10.1039/d0ob01726d. Org Biomol Chem. 2020. PMID: 33044473 Review.
-
Spiro-oxindoles as a Promising Class of Small Molecule Inhibitors of p53-MDM2 Interaction Useful in Targeted Cancer Therapy.Top Curr Chem (Cham). 2017 Feb;375(1):3. doi: 10.1007/s41061-016-0089-0. Epub 2016 Dec 9. Top Curr Chem (Cham). 2017. PMID: 27943171 Review.
References
-
-
For Selected reviews, see
- Cragg G. M. Newman D. J. Snader K. M. J. Nat. Prod. 1997;60:52. doi: 10.1021/np9604893. - DOI - PubMed
- Rishton G. M. Am. J. Cardiol. 2008;101:43. doi: 10.1016/j.amjcard.2008.02.007. - DOI - PubMed
- Harvey A. L. Drug Discov. Today. 2008;13:894. doi: 10.1016/j.drudis.2008.07.004. - DOI - PubMed
-
-
- Min-Chao T. Xuan C. Jun L. Rong H. Haiyan T. Chun-Jiang W. Angew. Chem., Int. Ed. 2014;5:4680.
- Elisabetta R. Giorgio A. Monica D. Marco N. Andrea P. Valentina P. Org. Biomol. Chem. 2016;14:6095. doi: 10.1039/C6OB00672H. - DOI - PubMed
- Wang H. Reisman S. E. Angew. Chem., Int. Ed. 2014;53:6206. doi: 10.1002/anie.201402571. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

