A Randomized, Double-Blind, Sham-Controlled Trial of Transcranial Direct Current Stimulation for the Treatment of Persistent Postural-Perceptual Dizziness (PPPD)
- PMID: 35493817
- PMCID: PMC9046552
- DOI: 10.3389/fneur.2022.868976
A Randomized, Double-Blind, Sham-Controlled Trial of Transcranial Direct Current Stimulation for the Treatment of Persistent Postural-Perceptual Dizziness (PPPD)
Abstract
Background: Persistent postural-perceptual dizziness (PPPD) is a functional vestibular disorder that causes chronic dizziness interfering with daily activities. Transcranial direct current stimulation (tDCS) has reportedly improved dizziness in patients with phobic postural vertigo in an open-label trial. However, no randomized, double-blind, sham-controlled study has been conducted on its therapeutic efficacy in PPPD.
Objective: This study was conducted to investigate the efficacy and safety of tDCS as an add-on treatment to pharmacotherapy in patients with PPPD. In addition, functional neuroimaging was used to identify the neural mechanisms underlying the effects of tDCS.
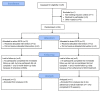
Materials and methods: In a randomized, double-blind, sham-controlled trial, 24 patients diagnosed with PPPD were randomized to receive active (2 mA, 20 min) or sham tDCS to the left dorsolateral prefrontal cortex (DLPFC), administered in 15 sessions over 3 weeks. The clinical measures that assess the severity of dizziness, depression, and anxiety were collected at baseline, immediate follow-up, 1-month follow-up, and 3-month follow-up. Adverse events were also observed. The effect of tDCS on regional cerebral blood flow (rCBF) was evaluated with single photon emission tomography before and after tDCS sessions.
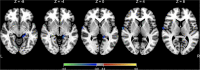
Results: For the primary outcome measure of the Dizziness Handicap Inventory (DHI) score, a significant main effect of time was found, but neither the treatment-by-time interaction effect nor the main effect of treatment was significant. For the Hamilton Depression Rating Scale (HDRS) score, there was a statistical significance for the treatment-by-time interaction effect and the main effect of time, but not for the main effect of treatment. However, the treatment-by-time interaction effect and the main effect of time on HDRS score appear to be due to one data point, an increase in depressive symptoms reported by the sham group at the 3-month follow-up. For the Activities-specific Balance Confidence (ABC) Scale and the Hamilton Anxiety Rating Scale scores, there were no significant main effects of time, treatment, and treatment-by-time interaction. In a comparison with the changes in rCBF between the groups, a significant treatment-by-time interaction effect was found in the right superior temporal and left hippocampus, controlling for age and sex.
Conclusion: Active tDCS was not found to be significantly more efficacious than sham tDCS on dizziness symptoms in patients with PPPD. It is conceivable that tDCS targeting the DLPFC may not be an optimal treatment option for reducing dizziness symptoms in PPPD. Our findings encourage further investigation on the effects of tDCS in PPPD, which considers different stimulation protocols in terms of stimulation site or the number of sessions.
Clinical trial registration: cris.nih.go.kr, identifier: KCT0005068.
Keywords: dizziness; neuromodulation; persistent postural-perceptual dizziness; single photon emission computed tomography; transcranial direct current stimulation.
Copyright © 2022 Im, Na, Kang, Jeong, Lee, Lee, Ahn, Chung and Song.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

