Development of novel spectroscopic and machine learning methods for the measurement of periodic changes in COVID-19 antibody level
- PMID: 35493849
- PMCID: PMC9040476
- DOI: 10.1016/j.measurement.2022.111258
Development of novel spectroscopic and machine learning methods for the measurement of periodic changes in COVID-19 antibody level
Abstract
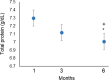
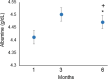
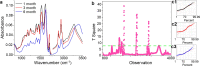
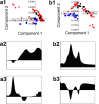
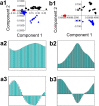
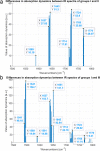
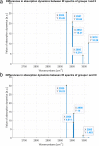
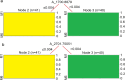
In this research, blood samples of 47 patients infected by COVID were analyzed. The samples were taken on the 1st, 3rd and 6th month after the detection of COVID infection. Total antibody levels were measured against the SARS-CoV-2 N antigen and surrogate virus neutralization by serological methods. To differentiate COVID patients with different antibody levels, Fourier Transform InfraRed (FTIR) and Raman spectroscopy methods were used. The spectroscopy data were analyzed by multivariate analysis, machine learning and neural network methods. It was shown, that analysis of serum using the above-mentioned spectroscopy methods allows to differentiate antibody levels between 1 and 6 months via spectral biomarkers of amides II and I. Moreover, multivariate analysis showed, that using Raman spectroscopy in the range between 1317 cm-1 and 1432 cm-1, 2840 cm-1 and 2956 cm-1 it is possible to distinguish patients after 1, 3, and 6 months from COVID with a sensitivity close to 100%.
Keywords: Anti-SARS-CoV-2 antibody levels; FTIR; Machine learning; Raman spectroscopy.
© 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X.i., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. - PMC - PubMed
-
- National SARS-CoV-2 Serology Assay Evaluation Group. Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet Infect Dis. 12 (2020) 1390-1400. https://doi.org/10.1016/S1473-3099(20)30634-4. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
