A Neuron, Microglia, and Astrocyte Triple Co-culture Model to Study Alzheimer's Disease
- PMID: 35493929
- PMCID: PMC9048896
- DOI: 10.3389/fnagi.2022.844534
A Neuron, Microglia, and Astrocyte Triple Co-culture Model to Study Alzheimer's Disease
Abstract
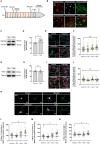
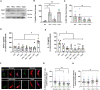
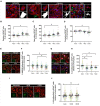
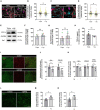
Glial cells are essential to understand Alzheimer's disease (AD) progression, given their role in neuroinflammation and neurodegeneration. There is a need for reliable and easy to manipulate models that allow studying the mechanisms behind neuron and glia communication. Currently available models such as co-cultures require complex methodologies and/or might not be affordable for all laboratories. With this in mind, we aimed to establish a straightforward in vitro setting with neurons and glial cells to study AD. We generated and optimized a 2D triple co-culture model with murine astrocytes, neurons and microglia, based on sequential seeding of each cell type. Immunofluorescence, western blot and ELISA techniques were used to characterize the effects of oligomeric Aβ (oAβ) in this model. We found that, in the triple co-culture, microglia increased the expression of anti-inflammatory marker Arginase I, and reduced pro-inflammatory iNOS and IL-1β, compared with microglia alone. Astrocytes reduced expression of pro-inflammatory A1 markers AMIGO2 and C3, and displayed a ramified morphology resembling physiological conditions. Anti-inflammatory marker TGF-β1 was also increased in the triple co-culture. Lastly, neurons increased post-synaptic markers, and developed more and longer branches than in individual primary cultures. Addition of oAβ in the triple co-culture reduced synaptic markers and increased CD11b in microglia, which are hallmarks of AD. Consequently, we developed a straightforward and reproducible triple co-cultured model, where cells resemble physiological conditions better than in individual primary cultures: microglia are less inflammatory, astrocytes are less reactive and neurons display a more mature morphology. Moreover, we are able to recapitulate Aβ-induced synaptic loss and CD11b increase. This model emerges as a powerful tool to study neurodegeneration and neuroinflammation in the context of AD and other neurodegenerative diseases.
Keywords: Alzheimer; astrocyte; co-culture; in vitro; inflammation; microglia; neuron; synapse.
Copyright © 2022 Luchena, Zuazo-Ibarra, Valero, Matute, Alberdi and Capetillo-Zarate.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Alberdi E., Sánchez-Gómez M. V., Ruiz A., Cavaliere F., Ortiz-Sanz C., Quintela-López T., et al. (2018). Mangiferin and morin attenuate oxidative stress, mitochondrial dysfunction, and neurocytotoxicity, induced by Amyloid Beta Oligomers. Oxid. Med. Cell. Longev. 2018:2856063. 10.1155/2018/2856063 - DOI - PMC - PubMed
-
- Alberdi E., Wyssenbach A., Alberdi M., Sánchez-Gómez M. V., Cavaliere F., Rodríguez J. J., et al. (2013). Ca 2+ -dependent endoplasmic reticulum stress correlates with astrogliosis in oligomeric amyloid β-treated astrocytes and in a model of Alzheimer’s disease. Aging Cell 12 292–302. 10.1111/acel.12054 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

