Canaloplasty and Trabeculotomy Combined with Phacoemulsification for Glaucoma: 12-Month Results of the GEMINI Study
- PMID: 35493971
- PMCID: PMC9039153
- DOI: 10.2147/OPTH.S362932
Canaloplasty and Trabeculotomy Combined with Phacoemulsification for Glaucoma: 12-Month Results of the GEMINI Study
Abstract
Purpose: To report 12-month efficacy outcomes of 360° canaloplasty and 180° trabeculotomy using the OMNI surgical system in combination with phacoemulsification in patients with mild-moderate open-angle glaucoma (OAG) and visually significant cataract.
Setting: Fifteen multi-subspecialty ophthalmology practices and surgery centers located in 14 US states.
Design: Prospective, multicenter, IRB approved study of patients treated with canaloplasty (360°) and trabeculotomy (180°). Eligible patients had cataract and mild-moderate OAG with intraocular pressure (IOP) ≤33 mmHg on 1 to 4 hypotensive medications. Unmedicated post-washout mean diurnal IOP (DIOP) ≥21 and ≤36 mmHg.
Methods: Medication washout preoperatively and prior to month 12 DIOP. Effectiveness outcomes were IOP and IOP lowering medication use. Safety outcomes included adverse events and secondary surgical interventions (SSIs). Evaluations at 1, 3, 6, and 12 months.
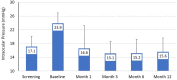
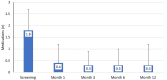
Results: A total of 149 subjects underwent surgery and 120 were included in the final effectiveness analysis. Mean (standard deviation) unmedicated diurnal IOP was reduced from 23.8 (3.1) mmHg at baseline to 15.6 (4.0) at month 12 (-35%) and medications (before washout) were reduced from 1.8 (0.9) at baseline to 0.4 (0.9) at month 12 (-80%). At month 12, 84.2% of eyes achieved IOP reductions >20% from baseline, 80% of eyes were medication-free, and 76% of eyes achieved IOP between 6-18 mmHg inclusive. Adverse events were uncommon. Most were mild and self-limited including transient hyphema (9 of 149; 6%) and transient IOP elevations (3 of 149; 2.0%). No eyes required SSIs or experienced loss of VA that was attributable to the device or procedure.
Conclusion: Canaloplasty and trabeculotomy performed with the OMNI surgical system at the time of phacoemulsification significantly reduces unmedicated mean diurnal IOP and medication use 12 months postoperatively, with an excellent safety profile. This procedure should be considered for eyes with mild-moderate OAG to reduce IOP, medication burden, or both.
Keywords: MIGS; OMNI; canaloplasty; glaucoma surgery; open-angle glaucoma; trabeculotomy; viscodilation.
© 2022 Gallardo et al.
Conflict of interest statement
MJG, SRS, SDV, IPS are consultants and speakers for Sight Sciences. SRS has an equity interest in Sight Sciences and reports grants and/or personal fees from Alcon, Aerie, Bausch & Lomb, Elios, IStar, Allysta Pharmaceuticals, Allergan/AbbVie, BVI, Glaukos, ICare, Katena, MST, Santen, grants/personal fees with equity from Ocular Science, Ocular Therapeutix and Sight Sciences, during the conduct of the study. SDV also reports personal fees from Alcon, Allergan, Bausch & Lomb, Carl Zeiss Meditec, Glaukos, Iridex, iStar Medical, Ivantis, Sight Sciences, Volk Optical, Santen, and Alphaeon, outside the submitted work; In addition, SDV has a patent Gonio lens system (8851676) issued; fasteners/deployment systems for ophthalmic tissue closure (13/434,562, 13/709,375, 14/046,488) pending. MFP reports research support from Sight Sciences, during the conduct of the study and is speakers bureau for Omeros, outside the submitted work. AC reports study fees for participation as principal investigator from Sight Sciences, during the conduct of the study. BF reports grants and/or personal fees from Alcon, Glaukos, Sight Sciences, grants, New World Medical, iStar Medical, Santen, and NiCox, during the conduct of the study. KD is an employee of Sight Sciences, Inc. The authors report no other conflicts of interest in this work.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

