New 1 H-benzimidazole-2-yl hydrazones with combined antiparasitic and antioxidant activity
- PMID: 35494105
- PMCID: PMC9044521
- DOI: 10.1039/d1ra07419a
New 1 H-benzimidazole-2-yl hydrazones with combined antiparasitic and antioxidant activity
Abstract
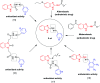
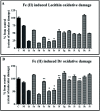
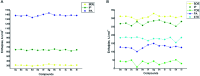
Parasitic infections, caused mainly by the species Trichinella spiralis (T. spiralis), are widespread around the world and lead to morbidity and mortality in the population. Meanwhile, some studies have showed that these parasites induce oxidative stress in the infected host. With the aim of developing a class of compounds combining anthelmintic with antioxidant properties, a series of new benzimidazolyl-2-hydrazones 5a-l, bearing hydroxyl- and methoxy-groups, were synthesized. The anthelmintic activity on encapsulated T. spiralis was studied in vitro thus indicating that all hydrazones were more active than the clinically used anthelmintic drugs albendazole and ivermectin. 5b and 5d killed the total parasitic larvae (100% effectiveness) after 24 hours incubation period at 37 °C in both concentrations (50 and 100 μg ml-1). The antioxidant activity of the target compounds was elucidated in vitro against stable free radicals DPPH and ABTS as well as iron induced oxidative damage in model systems containing biologically relevant molecules lecithin and deoxyribose. The two 2,3- and 3,4-dihydroxy hydrazones 5b and 5d were the most effective radical scavengers in all studied systems. DFT calculations were applied to calculate the reaction enthalpies in polar and nonpolar medium and estimate the preferred mechanism of antioxidant activity. The relative radical scavenging ability of compounds 5a-l showed a good correlation to the experimentally observed trends. It was found that the studied compounds are capable to react with various free radicals - ˙OCH3, ˙OOH and ˙OOCH3, through several possible reaction pathways - HAT in nonpolar medium, SPLET in polar medium and RAF in both media.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that there is no conflict of interests related to this study.
Figures










Similar articles
-
1H-benzimidazole-2-yl hydrazones as tubulin-targeting agents: Synthesis, structural characterization, anthelmintic activity and antiproliferative activity against MCF-7 breast carcinoma cells and molecular docking studies.Chem Biol Interact. 2021 Aug 25;345:109540. doi: 10.1016/j.cbi.2021.109540. Epub 2021 Jun 15. Chem Biol Interact. 2021. PMID: 34139148
-
Evaluation of the combined activity of benzimidazole arylhydrazones as new anti-Parkinsonian agents: monoamine oxidase-B inhibition, neuroprotection and oxidative stress modulation.Neural Regen Res. 2021 Nov;16(11):2299-2309. doi: 10.4103/1673-5374.309843. Neural Regen Res. 2021. PMID: 33818516 Free PMC article.
-
Synthesis, in vitro safety and antioxidant activity of new pyrrole hydrazones.Acta Pharm. 2020 Sep 1;70(3):303-324. doi: 10.2478/acph-2020-0026. Acta Pharm. 2020. PMID: 32074071
-
Considerations on the mechanism of action of artemisinin antimalarials: part 1--the 'carbon radical' and 'heme' hypotheses.Infect Disord Drug Targets. 2013 Aug;13(4):217-77. doi: 10.2174/1871526513666131129155708. Infect Disord Drug Targets. 2013. PMID: 24304352 Review.
-
Synthesis, characterization, computational, antioxidant and fluorescence properties of novel 1,3,5-trimesic hydrazones derivatives.Heliyon. 2021 Sep 25;7(9):e08074. doi: 10.1016/j.heliyon.2021.e08074. eCollection 2021 Sep. Heliyon. 2021. PMID: 34622075 Free PMC article. Review.
Cited by
-
Scaffolds imparting anthelmintic activity: recent advancements and SAR studies.Mol Divers. 2025 Feb;29(1):783-816. doi: 10.1007/s11030-024-10869-x. Epub 2024 Jul 31. Mol Divers. 2025. PMID: 39083219 Review.
-
Benzimidazoles Containing Piperazine Skeleton at C-2 Position as Promising Tubulin Modulators with Anthelmintic and Antineoplastic Activity.Pharmaceuticals (Basel). 2023 Oct 25;16(11):1518. doi: 10.3390/ph16111518. Pharmaceuticals (Basel). 2023. PMID: 38004384 Free PMC article.
-
Biological assessments of novel ultrasound-synthesized 2-arylbenzimidazole derivatives: antiproliferative and antibacterial effects.RSC Med Chem. 2025 Apr 30;16(7):3197-3212. doi: 10.1039/d5md00106d. eCollection 2025 Jul 16. RSC Med Chem. 2025. PMID: 40352670 Free PMC article.
-
Fused Triazinobenzimidazoles Bearing Heterocyclic Moiety: Synthesis, Structure Investigations, and In Silico and In Vitro Biological Activity.Molecules. 2023 Jun 27;28(13):5034. doi: 10.3390/molecules28135034. Molecules. 2023. PMID: 37446695 Free PMC article.
-
Novel Benzimidazole-Endowed Chalcones as α-Glucosidase and α-Amylase Inhibitors: An Insight into Structural and Computational Studies.Molecules. 2024 Nov 27;29(23):5599. doi: 10.3390/molecules29235599. Molecules. 2024. PMID: 39683757 Free PMC article.
References
-
- Trichinellosis, Annual Epidemiological Report for 2017, https://www.ecdc.europa.eu/en/publications-data/trichinellosis-annual-ep...
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

