Revisiting salicylidene-based anion receptors
- PMID: 35494355
- PMCID: PMC9043535
- DOI: 10.1039/d1ra07677a
Revisiting salicylidene-based anion receptors
Abstract
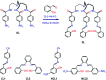
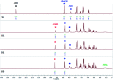
Several salicylidene-based colorimetric and fluorimetric anion sensors are known in the literature. However, our 1H-NMR experimental results (in DMSO-d6) showed hydrolysis of imine (-N[double bond, length as m-dash]CH-) bonds in salicylidene-based receptors (SL, CL1 and CL2) in the presence of quaternary ammonium salts (n-Bu4N+) of halides (Cl- and Br-) and oxo-anions (H2PO4 -, HSO4 - and CH3COO-). The mono-salicylidene compound CL1 showed the most extensive -N[double bond, length as m-dash]CH- bond hydrolysis in the presence of anions. In contrast, the di-salicylidene compound CL2 and the tris-salicylidene compound SL showed comparatively slow hydrolysis of -N[double bond, length as m-dash]CH- bonds in the presence of anions. Anion-induced imine bond cleavage in salicylidene compounds could easily be detected in 1H-NMR due to the appearance of the salicylaldehyde -CHO peak at 10.3 ppm which eventually became more intense over time, and the -N[double bond, length as m-dash]CH- peak at 8.9-9.0 ppm became considerably weaker. Furthermore, the formation of the salicylidene O-H⋯X- (X- = Cl-/Br-) hydrogen-bonded complex, peak broadening due to proton-exchange processes and keto-enol tautomerism have also been clearly observed in the 1H-NMR experiments. Control 1H-NMR experiments revealed that the presence of moisture in the organic solvents could result in gradual hydrolysis of the salicylidene compounds, and the rate of hydrolysis has further been enhanced significantly in the presence of an anion. Based on 1H-NMR results, we have proposed a general mechanism for the anion-induced hydrolysis of imine bonds in salicylidene-based receptors.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures








References
-
- Liu X. Hamon J.-R. Coord. Chem. Rev. 2019;389:94–118. doi: 10.1016/j.ccr.2019.03.010. - DOI
- Karmakar M. Chattopadhyay S. J. Mol. Struct. 2019;1186:155–186. doi: 10.1016/j.molstruc.2019.02.091. - DOI
- Zhang J. Xu L. Wong W.-Y. Coord. Chem. Rev. 2018;355:180–198. doi: 10.1016/j.ccr.2017.08.007. - DOI
- da Silva C. M. da Silva D. L. Modolo L. V. Alves R. B. de Resende M. A. Martins C. V. B. de Fátima A. J. Adv. Res. 2011;2:1–8. doi: 10.1016/j.jare.2010.05.004. - DOI
- Nath M. Saini P. K. Dalton Trans. 2011;40:7077–7121. doi: 10.1039/C0DT01426E. - DOI - PubMed
- Zoubi W. A. Mohamed S. G. Al-Hamdani A. A. S. Mahendradhany A. P. Ko Y. G. RSC Adv. 2018;8:23294–23318. doi: 10.1039/C8RA01890A. - DOI - PMC - PubMed
-
- Cozzi P. G. Chem. Soc. Rev. 2004;33:410–421. doi: 10.1039/B307853C. - DOI - PubMed
-
, and references therein;
- Gupta K. C. Sutar A. C. Coord. Chem. Rev. 2008;252:1420–1450. doi: 10.1016/j.ccr.2007.09.005. - DOI
- Drozdzak R. Allaert B. Ledoux N. Dragutan I. Dragutan V. Verpoort F. Adv. Synth. Catal. 2005;347:1721–1743. doi: 10.1002/adsc.200404389. - DOI
- Adaeo P. Kuznetsov M. L. Barroso S. Martins A. M. Avecilla F. Pessoa J. C. Inorg. Chem. 2012;51:11430–11449. doi: 10.1021/ic301153p. - DOI - PubMed
- Das P. Linert W. Coord. Chem. Rev. 2016;311:1–23. doi: 10.1016/j.ccr.2015.11.010. - DOI
- Gong D. Wang B. Jia X. Zhang X. Dalton Trans. 2014;43:4169–4178. doi: 10.1039/C3DT52708E. - DOI - PubMed
-
- Che C. M. Chan S. C. Xiang H. F. Chan M. C. Liu Y. Wang Y. Chem. Commun. 2004:1484–1485. doi: 10.1039/B402318H. - DOI - PubMed
- Sano T. Nishio Y. Hamada Y. Takahashi H. Usuki T. Shibata K. J. Mater. Chem. 2000;10:157–161. doi: 10.1039/A903239H. - DOI
- Li S. H. Chen F. R. Zhou Y. F. Wang J. N. Zhang H. Xu J. G. Chem. Commun. 2009:4179–4181. doi: 10.1039/B906467B. - DOI - PubMed
- Lee S. A. You G. R. Choi Y. W. Jo H. Y. Kim A. R. Noh I. Kim S. J. Kim Y. Kim C. Dalton Trans. 2014;43:6650–6659. doi: 10.1039/C4DT00361F. - DOI - PubMed
-
- Sun Y. Lu Y. Bian M. Yang Z. Ma X. Liu W. Eur. J. Med. Chem. 2021;211:113098. doi: 10.1016/j.ejmech.2020.113098. - DOI - PubMed
- Parveen S. Appl. Organomet. Chem. 2020;34:e5687. doi: 10.1002/aoc.5687. - DOI - PubMed
- More M. S. Joshi P. G. Mishra Y. K. Khanna P. K. Mater. Today Chem. 2019;14:100195. doi: 10.1016/j.mtchem.2019.100195. - DOI - PMC - PubMed
- Kaczmarek M. T. Zabiszak M. Nowak M. Jastrzab R. Coord. Chem. Rev. 2018;370:42–54. doi: 10.1016/j.ccr.2018.05.012. - DOI
- Hajrezaie M. Paydar M. Looi C. Y. Moghadamtousi S. Z. Hassandarvish P. Salga M. S. Karimian H. Shams K. Zahedifard M. Majid N. A. Ali H. M. Abdulla M. A. Sci. Rep. 2015;5:9097–9104. doi: 10.1038/srep09097. - DOI - PMC - PubMed
-
- Bowman-James K., Bianchi A. and Garcia-Espana E., Anion Coordination Chemistry, Wiley-VCH, 1st edn, 2011
LinkOut - more resources
Full Text Sources

