Development of novel, biocompatible, polyester amines for microglia-targeting gene delivery
- PMID: 35494387
- PMCID: PMC9043621
- DOI: 10.1039/d1ra06277h
Development of novel, biocompatible, polyester amines for microglia-targeting gene delivery
Abstract
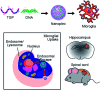
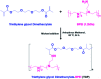
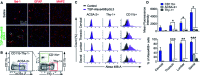
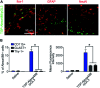
Recent progress in personalized medicine and gene delivery has created exciting opportunities in therapeutics for central nervous system (CNS) disorders. Despite the interest in gene-based therapies, successful delivery of nucleic acids for treatment of CNS disorders faces major challenges. Here we report the facile synthesis of a novel, biodegradable, microglia-targeting polyester amine (PEA) carrier based on hydrophilic triethylene glycol dimethacrylate (TG) and low-molecular weight polyethylenimine (LMW-PEI). This nanocarrier, TG-branched PEI (TGP), successfully condensed double-stranded DNA into a size smaller than 200 nm. TGP nanoplexes were nontoxic in primary mixed glial cells and showed elevated transfection efficiency compared with PEI-25K and lipofector-EZ. After intrathecal and intracranial administration, PEA nanoplexes delivered genes specifically to microglia in the spinal cord and brain, respectively, proposing TGP as a novel microglia-specific gene delivery nanocarrier. The microglia-specific targeting of the TGP nanocarrier offers a new therapeutic strategy to modulate CNS disorders involving aberrant microglia activation while minimizing off-target side effects.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

