Strategies for Glycoengineering Therapeutic Proteins
- PMID: 35494652
- PMCID: PMC9043614
- DOI: 10.3389/fchem.2022.863118
Strategies for Glycoengineering Therapeutic Proteins
Abstract
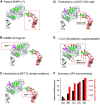
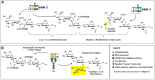
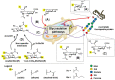
Almost all therapeutic proteins are glycosylated, with the carbohydrate component playing a long-established, substantial role in the safety and pharmacokinetic properties of this dominant category of drugs. In the past few years and moving forward, glycosylation is increasingly being implicated in the pharmacodynamics and therapeutic efficacy of therapeutic proteins. This article provides illustrative examples of drugs that have already been improved through glycoengineering including cytokines exemplified by erythropoietin (EPO), enzymes (ectonucleotide pyrophosphatase 1, ENPP1), and IgG antibodies (e.g., afucosylated Gazyva®, Poteligeo®, Fasenra™, and Uplizna®). In the future, the deliberate modification of therapeutic protein glycosylation will become more prevalent as glycoengineering strategies, including sophisticated computer-aided tools for "building in" glycans sites, acceptance of a broad range of production systems with various glycosylation capabilities, and supplementation methods for introducing non-natural metabolites into glycosylation pathways further develop and become more accessible.
Keywords: N-glycans; biomanufacturing; glycoengineering; glycosylation; pharmacodynamics; pharmacokinetics; therapeutic.
Copyright © 2022 Dammen-Brower, Epler, Zhu, Bernstein, Stabach, Braddock, Spangler and Yarema.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Agatemor C., Muthiah K., Ha L., Chai J., Osman A., Robertson B. M., et al. (2021). “Imaging Glycans with Metabolic Glycoengineering,” in Chemistry, Molecular Sciences and Chemical Engineering. Editor BarchiJr J. (Elsevier; ), 253–274. 10.1016/B978-0-12-409547-2.14962-5 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

