A novel cellulose/chitosan composite nanofiltration membrane prepared with piperazine and trimesoyl chloride by interfacial polymerization
- PMID: 35494724
- PMCID: PMC9047020
- DOI: 10.1039/c9ra09023a
A novel cellulose/chitosan composite nanofiltration membrane prepared with piperazine and trimesoyl chloride by interfacial polymerization
Abstract
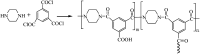
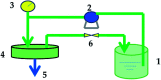
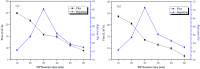
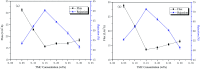
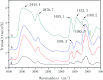
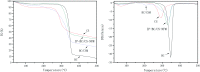
Bamboo cellulose (BC) is one of the most abundant renewable, hydrophilic, inexpensive, and biodegradable organic materials. The cellulose membrane is one of the best materials for replacing petroleum-based polymer films used for water purification. In this study, N-methylmorpholine-N-oxide (NMMO) was used as a solvent to dissolve cellulose and chitosan, and a regenerated cellulose/chitosan membrane (BC/CSM) was prepared by phase inversion. A new kind of cellulose/chitosan nanofiltration membrane (IP-BC/CS-NFM) was obtained by the interfacial polymerization of piperazine (PIP) and trimesoyl chloride (TMC). The IP-BC/CS-NFM was characterized by Fourier transform infrared spectroscopy (FT-IR), field emission scanning electron microscopy (FE-SEM), atomic force microscopy (AFM), thermal gravimetric analysis (TGA), the retention rate, and water flux. FT-IR analysis showed that polypiperazine amide was formed. Additionally, FE-SEM and AFM showed that a uniform roughness and dense functional layer was formed on the surface of the IP-BC/CS-NFM. Furthermore, TGA analysis showed that the thermal stability of IP-BC/CS-NFM is better than that of BC/CSM. The inorganic salt retention of IP-BC/CS-NFM was measured using a membrane performance evaluation instrument, following the order R(Na2SO4) > R(MgSO4) > R(MgCl2) > R(NaCl). At a pressure of 0.5 MPa, the retention rates for NaCl, Na2SO4, MgSO4, MgCl2, Methyl Orange, and Methyl Blue were 40.26%, 71.34%, 62.55%, 53.28%, 93.65%, and 98.86%, and the water flux values were 15.64, 13.56, 14.03, 14.88, 13.28, and 12.35 L m-2 h-1, respectively. The IP-BC/CS-NFM showed better water flux and a higher rejection rate in aqueous dye-salt solutions, and had a good separation performance under different operating pressure conditions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







References
-
- Wang J. Huang T. F. Zhang L. Yu Q. J. Hou L. A. Environ. Technol. 2017;39(23):1–28. - PubMed
-
- Li S. S. Luo J. Q. Wan Y. H. J. Membr. Sci. 2018;549:120–128. doi: 10.1016/j.memsci.2017.11.075. - DOI
-
- Huang T. F. Niu Y. B. Zhang F. Zhang L. Chen S. F. Yu Q. J. Applied Materials Today. 2017;9:176–183. doi: 10.1016/j.apmt.2017.07.003. - DOI
-
- Lv Y. Du Y. Chen Z. X. Qiu W. Z. Xu Z. K. J. Membr. Sci. 2018;545:99–106. doi: 10.1016/j.memsci.2017.09.066. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

