Three-dimensional thiophene-diketopyrrolopyrrole-based molecules/graphene aerogel as high-performance anode material for lithium-ion batteries
- PMID: 35494733
- PMCID: PMC9043015
- DOI: 10.1039/d1ra06528a
Three-dimensional thiophene-diketopyrrolopyrrole-based molecules/graphene aerogel as high-performance anode material for lithium-ion batteries
Abstract
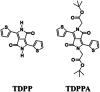
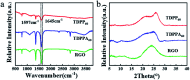
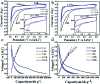
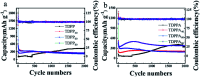
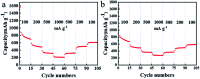
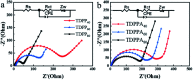
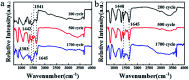
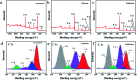
Herein, 3,6-di(thiophen-2-yl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione (TDPP) and di-tert-butyl 2,2'-(1,4-dioxo-3,6-di(thiophen-2-yl)pyrrolo[3,4-c]pyrrole-2,5(1H,4H)-diyl)diacetate (TDPPA) were synthesized, which were then loaded in graphene aerogels. The as-prepared thiophene-diketopyrrolopyrrole-based molecules/reduced graphene oxide composites for lithium-ion battery (LIB) anode composites consist of DPPs nanorods on a graphene network. In relation to the DPPs part, embedding DPPs nanorods into graphene aerogels can effectively reduce the dissolution of DPPs in the electrolyte. It can serve to prevent electrode rupture and improve electron transport and lithium-ion diffusion rate, by partially connecting DPPs nanorods through graphene. The composite not only has a high reversible capacity, but also shows excellent cycling stability and performance, due to the densely distributed graphene nanosheets forming a three-dimensional conductive network. The TDPP60 electrode exhibits high reversible capacity and excellent performance, showing an initial discharge capacity of 835 mA h g-1 at a current density of 100 mA g-1. Even at a current density of 1000 mA g-1, after 500 cycles, it still demonstrates a discharge capacity of 303 mA h g-1 with a capacity retention of 80.7%.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
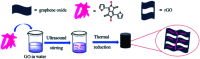

References
LinkOut - more resources
Full Text Sources

