Novel potentiometric sensors for determination of ondansetron hydrochloride in pure and dosage form
- PMID: 35494734
- PMCID: PMC9042692
- DOI: 10.1039/d1ra03268b
Novel potentiometric sensors for determination of ondansetron hydrochloride in pure and dosage form
Abstract
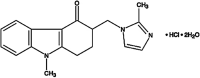
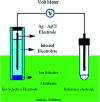
A new and sensitive potentiometric method has been developed and characterized for four novel sensors responsive to ondansetron hydrochloride. The potentiometric sensor method includes advancement of ondansetron hydrochloride sensors using a membrane comprised of molybdophosphoric acid (MPA) and ondansetron as an electro-active material in a polyvinylchloride (PVC) matrix membrane plasticized with di-butyl phthalate (DBPH), ortho-nitrophenyloctyl ether (O-NPOE), di-octyl phthalate (DOPH), or di-butyl phosphate (DBP). The validity of sensors in the present work has been examined, and steady and reproducible responses were obtained over the concentration ranges of 7.3 × 10-5 to 1.0 × 10-2, 6.6 × 10-6 to 1.0 × 10-2, 1.0 × 10-5 to 1.0 × 10-2, and 2.0 × 10-5 to 1.0 × 10-2 M for DBPH-, O-NPOE-, DOPH-, and DBP-ondansetron, respectively. The sensors revealed Nernstian gradients of 59.61 ± 0.50, 57.71 ± 0.23, 53.01 ± 0.14, and 53.20 ± 0.35 mV per decade individually with pH ranges of 2.5-5.5 in DBPH and 3.5-5.0 in O-NPOE electrodes, and 4.0-5.5 for both individual DOPH and DBP plasticized film-based sensors. The time responses for the sensors were 30, 32, 31, and 29 s for DBPH-, O-NPOE-, DOPH-, and DBP-ondansetron, respectively. The developed sensors also exhibited high selectivity towards ondansetron hydrochloride against different interfering species of inorganic particles with long-term stability of approximately 41, 36, 18, and multiple days for the DBPH, O-NPOE, DOPH, and DBP electrodes.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Asad R. Subhan I. A. Aslam K. Rehman A. Application of certain π-acceptors for the spectrophotometric determination of ondansetron in pharmaceutical formulations. Anal. Chem. 2007;6(2):43–47.
-
- British pharmacopoeia, Version 17, Copyright by, London, 2012
-
- British Pharmacopoeia, Her Majesty, Stationery Office, London, 2003, 2
-
- S. Mujbaile, P. Prasad and S. Wate, Simultaneous estimation of Ondansetron and Pantoprazole in solid dosage form by first derivative spectroscopy method, Tablet 4, no. 40, 2012, 100–117
-
- Zaabal M. Doulache M. Bakirhan N. K. Kaddour S. Saidat B. Ozkan S. A. A facile strategy for construction of sensor for detection of ondansetron and investigation of its redox behavior and thermodynamic parameters. Electroanalysis. 2019;31(7):1279–1290. doi: 10.1002/elan.201800658. - DOI
LinkOut - more resources
Full Text Sources

