Mitochondrial Transplantation Attenuates Neural Damage and Improves Locomotor Function After Traumatic Spinal Cord Injury in Rats
- PMID: 35495036
- PMCID: PMC9039257
- DOI: 10.3389/fnins.2022.800883
Mitochondrial Transplantation Attenuates Neural Damage and Improves Locomotor Function After Traumatic Spinal Cord Injury in Rats
Abstract
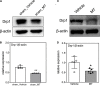
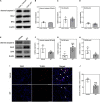
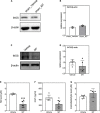
Mitochondrial dysfunction is a hallmark of secondary neuroinflammatory responses and neuronal death in spinal cord injury (SCI). Even though mitochondria-based therapy is an attractive therapeutic option for SCI, the efficacy of transplantation of allogeneic mitochondria in the treatment of SCI remains unclear. Herein, we determined the therapeutic effects of mitochondrial transplantation in the traumatic SCI rats. Compressive SCI was induced by applying an aneurysm clip on the T10 spinal cord of rats. A 100-μg bolus of soleus-derived allogeneic mitochondria labeled with fluorescent tracker was transplanted into the injured spinal cords. The results showed that the transplanted mitochondria were detectable in the injured spinal cord up to 28 days after treatment. The rats which received mitochondrial transplantation exhibited better recovery of locomotor and sensory functions than those who did not. Both the expression of dynamin-related protein 1 and severity of demyelination in the injured cord were reduced in the mitochondrial transplanted groups. Mitochondrial transplantation also alleviated SCI-induced cellular apoptosis and inflammation responses. These findings suggest that transplantation of allogeneic mitochondria at the early stage of SCI reduces mitochondrial fragmentation, neuroapoptosis, neuroinflammation, and generation of oxidative stress, thus leading to improved functional recovery following traumatic SCI.
Keywords: allogenic mitochondria; mitochondrial dysfunction; mitochondrial transplantation; oxidative stress; spinal cord injury.
Copyright © 2022 Lin, Fang, Hsu, Huang, Lee, Huang, Chen, Lam and Lee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

