Resonance Raman view of the active site architecture in bacterial DyP-type peroxidases
- PMID: 35495352
- PMCID: PMC9050505
- DOI: 10.1039/d0ra00950d
Resonance Raman view of the active site architecture in bacterial DyP-type peroxidases
Abstract
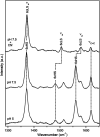
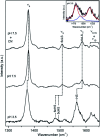
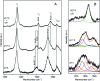
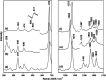
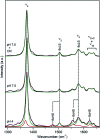
Dye decolorizing peroxidases (DyPs) are novel haem-containing peroxidases, which are structurally unrelated to classical peroxidases. They lack the highly conserved distal histidine that acts as an acid-base catalyst in the catalytic reaction of classical peroxidases, which implies distinct mechanistic properties. Despite the remarkable catalytic properties and recognized potential for biotechnology applications, the knowledge of DyP's structural features in solution, which govern the reactivity and catalysis, is lagging behind. Resonance Raman (RR) spectroscopy can reveal fine details of the active site structure in hemoproteins, reporting on the oxidation and spin state and coordination of the haem cofactor. We provide an overview of the haem binding pocket architecture of the enzymes from A, B and C DyP subfamilies, in the light of those established for classical peroxidases and search for subfamily specific features among DyPs. RR demonstrates that multiple spin populations typically co-exist in DyPs, like in the case of classical peroxidases. The haem spin/coordination state is strongly pH dependent and correlates well with the respective catalytic properties of DyPs. Unlike in the case of classical peroxidases, a surprisingly high abundance of catalytically incompetent low spin population is observed in several DyPs, and tentatively related to the alternative physiological function of these enzymes. The molecular details of active sites of DyPs, elucidated by RR spectroscopy, can furthermore guide approaches for biotechnological exploitation of these promising biocatalysts.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

