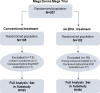
Evaluation of the Retinopathy of Prematurity Activity Scale (ROP-ActS) in a randomised controlled trial aiming for prevention of severe ROP: a substudy of the Mega Donna Mega trial
- PMID: 35495419
- PMCID: PMC8996016
- DOI: 10.1136/bmjophth-2021-000923
Evaluation of the Retinopathy of Prematurity Activity Scale (ROP-ActS) in a randomised controlled trial aiming for prevention of severe ROP: a substudy of the Mega Donna Mega trial
Abstract
Objective: The current grading of retinopathy of prematurity (ROP) does not sufficiently discriminate disease severity for evaluation of trial interventions. The published ROP Activity Scales (original: ROP-ActS and modified: mROP-ActS), describing increasing severity of ROP, versus the categorical variables severe ROP, stage, zone and plus disease were evaluated as discriminators of the effect of an ROP preventive treatment.
Methods and analysis: The Mega Donna Mega trial investigated ROP in infants born <28-week gestational age (GA), randomised to arachidonic acid (AA) and docosahexaenoic acid (DHA) supplementation or no supplementation. Of 207 infants, 86% with finalised ROP screening were included in this substudy. ROP-ActS versus standard variables were evaluated using Fisher's non-parametric permutation test, multivariable logistic and linear regression and marginal fractional response models.
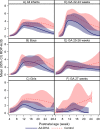
Results: The AA:DHA group (n=84) and the control group (n=93) were well balanced. The maximum ROP-ActS measurement was numerically but not significantly lower in the AA:DHA group (mean: 4.0 (95% CI 2.9 to 5.0)) versus the control group (mean: 5.3 (95% CI 4.1 to 6.4)), p=0.11. In infants with any ROP, the corresponding scale measurements were 6.8 (95% CI 5.4 to 8.2) and 8.7 (95% CI 7.5 to 10.0), p=0.039. Longitudinal profiles of the scale were visually distinguished for the categories of sex and GA for the intervention versus control.
Conclusions: The preventive effect of AA:DHA supplementation versus no supplementation was better discriminated by the trial's primary outcome, severe ROP, than by ROP-ActS. The sensitivity and the linear qualities of ROP-ActS require further validations on large data sets and perhaps modifications.
Trial registration number: NCT03201588.
Keywords: Diagnostic tests/Investigation; Retina; Treatment other; Vision.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Swedish national guidelines for screening and management of ROP 2021, 2022. Available: https://www.medscinet.com/ROP/uploads/Nationella%20guidelines%20ROP-revi... [Accessed 23 Feb 2022].
-
- Swedish national guidelines for screening and management of ROP 2012, 2022. Available: https://neo.barnlakarforeningen.se/wp-content/uploads/sites/14/2014/03/R... [Accessed 28 Jan 2022].
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
