Direct synthesis of sulfenamides, sulfinamides, and sulfonamides from thiols and amines
- PMID: 35495485
- PMCID: PMC9042206
- DOI: 10.1039/d1ra04368d
Direct synthesis of sulfenamides, sulfinamides, and sulfonamides from thiols and amines
Abstract
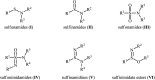
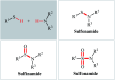
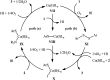
Needless to say that organosulfur compounds with sulfur-nitrogen bonds have found various applications in diverse fields such as pharmaceuticals, agrochemicals, polymers, and so forth. Three major groups of such compounds are sulfenamides, sulfinamides, and sulfonamides which have been widely applied as building blocks in medical chemistry. Owing to their significant role in drug design and discovery programs, the search for and development of efficient, environmentally friendly, and economic processes for the preparation of the title compounds is of great importance in the pharmaceutical industry. Recently, oxidative coupling of thiols and amines, two readily available low-cost commodity chemicals, has emerged as a highly useful method for synthesizing structurally diverse sulfenamides, sulfinamides, and sulfonamides in a single step. Since this strategy does not require additional pre-functionalization and de-functionalization steps, it considerably streamlines synthetic routes and substantially reduces waste generation. This review will focus on recent advances and achievements in this attractive research arena.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
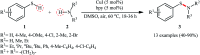

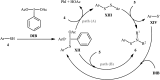
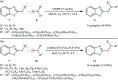
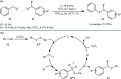


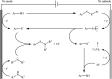












References
-
- Shimizu M. Suzuki S. Y. Tanaka S. Ando W. Sakai N. Phosphorus, Sulfur Silicon Relat. Elem. 2019;194:764–767. doi: 10.1080/10426507.2019.1603724. - DOI
- Majedi S. Majedi S. J. Chem. Lett. 2020;1:2–8.
-
- Craine L. Raban M. Chem. Rev. 1989;89:689–712. doi: 10.1021/cr00094a001. - DOI
- Klioze S. S. Allen R. C. Wilker J. C. Woodward D. L. J. Med. Chem. 1980;23:677–679. doi: 10.1021/jm00180a020. - DOI - PubMed
- Li Z. Shi Y. Zhu A. Zhao Y. Wang H. Binks B. P. Wang J. Angew. Chem., Int. Ed. 2021;60:3928–3933. doi: 10.1002/anie.202010750. - DOI - PubMed
- Zhang L. Zhang M. You S. Ma D. Zhao J. Chen Z. Sci. Total Environ. 2021;780:146505. doi: 10.1016/j.scitotenv.2021.146505. - DOI - PubMed
- Wang H. Cui J. Zhao Y. Li Z. Wang J. Green Chem. 2021;23:405–411. doi: 10.1039/D0GC03557B. - DOI
- Zhang L. Zheng J. Tian S. Zhang H. Guan X. Zhu S. Li Z. J. Environ. Sci. 2020;91:212–221. doi: 10.1016/j.jes.2020.02.010. - DOI - PubMed
- Wang X. Gao P. Liu Y. Li H. Lu F. Curr. Bioinf. 2020;15(10):493–502. doi: 10.2174/1574893615666200207094357. - DOI
- Niu M. Lin Y. Zou Q. Plant Mol. Biol. 2021;105(4–5):483–495. doi: 10.1007/s11103-020-01102-y. - DOI - PubMed
- Sun S. Xu L. Zou Q. Wang G. Gorodkin J. Bioinformatics. 2021;37:1319–1321. doi: 10.1093/bioinformatics/btaa832. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

