Liposome leakage and increased cellular permeability induced by guanidine-based oligomers: effects of liposome composition on liposome leakage and human lung epithelial barrier permeability
- PMID: 35495488
- PMCID: PMC9042049
- DOI: 10.1039/d1ra05478c
Liposome leakage and increased cellular permeability induced by guanidine-based oligomers: effects of liposome composition on liposome leakage and human lung epithelial barrier permeability
Abstract
Over the decades, guanidine-based oligomer groups have been one of the most widely used antimicrobial agents. Reportedly, these cationic oligomers cause serious damage to microorganisms but have low toxicity to humans. However, public concerns regarding the guanidine group have rapidly grown after the fatal misuse of these oligomers as humidifier disinfectants, which resulted in thousands of fatalities in South Korea. Herein, we investigated liposome leakage and cellular permeability changes caused by polyhexamethylene guanidine (PHMG) and polyhexamethylene biguanide (PHMB), both representative guanidine-based oligomers. The leakage of zwitterionic liposomes, induced by cationic oligomers, was more extensive than that of negative liposomes, indicating that oligomer adsorption onto lipid head groups via electrostatic interaction cannot fully explain the induced lipid membrane damage. Furthermore, lipid packing parameters, including intrinsic curvature, cholesterol content, and lipid phases, affected liposome leakage, particularly for PHMG. Cellular permeability tests were performed using an A549 cell monolayer model and a respiratory 3D tissue model, revealing that PHMG and PHMB damaged cell membranes and reduced cell barrier function. Furthermore, liposome leakage induced by PHMG and PHMB was higher in human lung surfactant-mimicking liposomes than that observed in Escherichia coli-mimicking liposomes. These results indicated that human cells are susceptible to guanidine-based oligomers. Considering that the interaction of oligomers and cell membranes is a major mechanism of toxicity initiation, this study provides crucial insights into the action of these disinfectants on mammalian cells.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




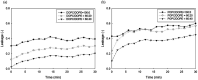

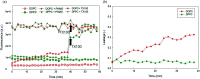

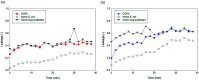
Similar articles
-
Guanidine-based disinfectants, polyhexamethylene guanidine-phosphate (PHMG-P), polyhexamethylene biguanide (PHMB), and oligo(2-(2-ethoxy)ethoxyethyl guanidinium chloride (PGH) induced epithelial-mesenchymal transition in A549 alveolar epithelial cells.Inhal Toxicol. 2019 Mar;31(4):161-166. doi: 10.1080/08958378.2019.1624896. Epub 2019 Jun 10. Inhal Toxicol. 2019. PMID: 31179775
-
Effects of lipid membrane composition on the distribution of biocidal guanidine oligomer with solid supported lipid membranes.RSC Adv. 2020 Jun 10;10(38):22343-22351. doi: 10.1039/d0ra03108a. eCollection 2020 Jun 10. RSC Adv. 2020. PMID: 35514581 Free PMC article.
-
Adsorption of CMIT/MIT on the Model Pulmonary Surfactant Monolayers.J Oleo Sci. 2024;73(4):437-444. doi: 10.5650/jos.ess23165. J Oleo Sci. 2024. PMID: 38556278
-
Adverse health effects of humidifier disinfectants in Korea: lung toxicity of polyhexamethylene guanidine phosphate.J Toxicol Sci. 2016;41(6):711-717. doi: 10.2131/jts.41.711. J Toxicol Sci. 2016. PMID: 27853099 Review.
-
[Concern about lung damage caused by guanidine cationic disinfectants].Zhonghua Yu Fang Yi Xue Za Zhi. 2020 Feb 6;54(2):121-123. doi: 10.3760/cma.j.issn.0253-9624.2020.02.001. Zhonghua Yu Fang Yi Xue Za Zhi. 2020. PMID: 32074695 Review. Chinese.
Cited by
-
Evolutionary and in silico guided development of novel peptide analogues for antibacterial activity against ESKAPE pathogens.Curr Res Microb Sci. 2023 Mar 17;4:100183. doi: 10.1016/j.crmicr.2023.100183. eCollection 2023. Curr Res Microb Sci. 2023. PMID: 37032813 Free PMC article.
-
Risk of non-cancer respiratory diseases attributed to humidifier disinfectant exposure in Koreans: age-period-cohort and differences-in-difference analyses.Epidemiol Health. 2025;47:e2025006. doi: 10.4178/epih.e2025006. Epub 2025 Feb 22. Epidemiol Health. 2025. PMID: 39999791 Free PMC article.
-
Toxicological evidence integration to confirm the biological plausibility of the association between humidifier disinfectant exposure and respiratory diseases using the AEP-AOP framework.Epidemiol Health. 2024;46:e2024060. doi: 10.4178/epih.e2024060. Epub 2024 Jul 7. Epidemiol Health. 2024. PMID: 39026433 Free PMC article.
References
-
- Chen A. Peng H. Blakey I. Whittaker A. K. Polym. Rev. 2017;57:276–310. doi: 10.1080/15583724.2016.1223131. - DOI
LinkOut - more resources
Full Text Sources

