Hierarchical hyper-branched titania nanorods with tuneable selectivity for CO2 photoreduction
- PMID: 35495501
- PMCID: PMC9041433
- DOI: 10.1039/d1ra05414g
Hierarchical hyper-branched titania nanorods with tuneable selectivity for CO2 photoreduction
Abstract
Utilising captured CO2 and converting it into solar fuels can be extremely beneficial in reducing the constantly rising CO2 concentration in the atmosphere while simultaneously addressing energy crisis issues. Hence, many researchers have focused their work on the CO2 photoreduction reaction for the last 4 decades. Herein, the titania hyper-branched nanorod (HBN) thin films, with a novel hierarchical dendritic morphology, revealed enhanced CO2 photoreduction performance. The HBNs exhibited enhanced photogenerated charge production (66%), in comparison with P25 (39%), due to the unique hyper-branched morphology. Furthermore, the proposed HBN thin films exhibited a high degree of control over the product selectivity, by undergoing a facile phase-altering treatment. The selectivity was shifted from 91% towards CO, to 67% towards CH4. Additionally, the HBN samples showed the potential to surpass the conversion rates of the benchmark P25 TiO2 in both CO and CH4 production. To further enhance the selectivity and overall performance of the HBNs, RuO2 was incorporated into the synthesis, which enhanced the CH4 selectivity from 67% to 74%; whereas the incorporation of CuO revealed a selectivity profile comparative to P25.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
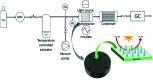


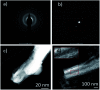
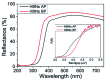
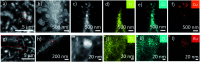
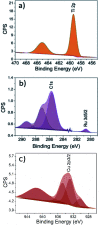
References
-
- Chang X. Wang T. Gong J. Energy Environ. Sci. 2016;9:2177–2196. doi: 10.1039/C6EE00383D. - DOI
-
- Liu L. Zhao C. Xu J. Li Y. Appl. Catal., B. 2015;179:489–499. doi: 10.1016/j.apcatb.2015.06.006. - DOI
-
- Osterloh F. E. ACS Energy Lett. 2017;2:445–453. doi: 10.1021/acsenergylett.6b00665. - DOI
-
- Thompson W. A. Sanchez Fernandez E. Maroto-Valer M. M. ACS Sustainable Chem. Eng. 2020;8:4677–4692. doi: 10.1021/acssuschemeng.9b06170. - DOI
-
- Shankar R. Sachs M. Francàs L. Lubert-Perquel D. Kerherve G. Regoutz A. Petit C. J. Mater. Chem. 2019;7:23931–23940. doi: 10.1039/C9TA02793A. - DOI
LinkOut - more resources
Full Text Sources

