Iron(iii) chelated paramagnetic polymeric nanoparticle formulation as a next-generation T 1-weighted MRI contrast agent
- PMID: 35495502
- PMCID: PMC9041822
- DOI: 10.1039/d1ra05544e
Iron(iii) chelated paramagnetic polymeric nanoparticle formulation as a next-generation T 1-weighted MRI contrast agent
Abstract
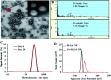
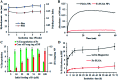
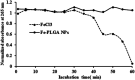
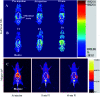
Magnetic resonance imaging (MRI) is a routinely used imaging technique in medical diagnostics. To enhance the quality of MR images, contrast agents (CAs) are used, which account for nearly 40% of MRI exams in the clinic globally. The most used CAs are gadolinium-based CAs (GBCAs) but the use of GBCAs has been linked with metal-deposition in vital organs. Gadolinium deposition has been shown to be correlated with nephrogenic systemic fibrosis, a fibrosis of the skin and internal organs. Therefore, there is an unmet need for a new CA alternative to GBCAs for T 1-weighted Ce-MRI. Herein, we designed paramagnetic ferric iron(iii) ion-chelated poly(lactic-co-glycolic)acid nanoparticle formulation and routinely examined their application in Ce-MRI using clinical and ultra-high-field MRI scanners. Nanoparticles were monodispersed and highly stable at physiological pH over time with the hydrodynamic size of 130 ± 12 nm and polydispersity index of 0.231 ± 0.026. The T 1-contrast efficacy of the nanoparticles was compared with commercial agent gadopentetate dimeglumine, called Magnevist®, in aqueous phantoms in vitro and then validated in vivo by visualizing an angiographic map in a clinical MRI scanner. Relaxivities of the nanoparticles in an aqueous environment were r 1 = 10.59 ± 0.32 mmol-1 s-1 and r 1 = 3.02 ± 0.14 mmol-1 s-1 at 3.0 T and 14.1 T measured at room temperature and pH 7.4, respectively. The clinically relevant magnetic field relaxivity is three times higher compared to the Magnevist®, a clinical GBCA, signifying its potential applicability in clinical settings. Moreover, iron is an endogenous metal with known metabolic safety, and the polymer and phospholipids used in the nanoconstruct are biodegradable and biocompatible components. These properties further put the proposed T 1 agent in a promising position in contrast-enhanced MRI of patients with any disease conditions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Ibrahim M. A., Hazhirkarzar B. and Dublin A. B., in StatPearls, StatPearls Publishing, Treasure Island (FL), 2020
-
- MRI in Practice, Wiley, 5th edn, https://www.wiley.com/en-us/MRI+in+Practice%2C+5th+Edition-p-9781119392002, accessed, April 21, 2020
-
- Hahn F. J. Chu W. K. Coleman P. E. Anderson J. C. Dobry C. A. Imray T. J. Hahn P. Y. Lee S. H. Radiol. Clin. North Am. 1988;26:717–735. - PubMed
-
- Marasini R. Nguyen T. D. T. Aryal S. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020;12:e1580. - PubMed
LinkOut - more resources
Full Text Sources

